PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs
- PMID: 16917540
- PMCID: PMC1550279
- DOI: 10.1172/JCI28057
PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs
Abstract
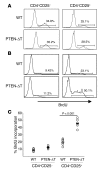
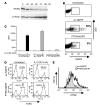
One of the greatest barriers against harnessing the potential of CD4+ CD25+ Tregs as a cellular immunotherapy is their hypoproliferative phenotype. We have previously shown that the hypoproliferative response of Tregs to IL-2 is associated with defective downstream PI3K signaling. Here, we demonstrate that targeted deletion of the lipid phosphatase PTEN (phosphatase and tensin homolog deleted on chromosome 10) regulates the peripheral homeostasis of Tregs in vivo and allows their expansion ex vivo in response to IL-2 alone. PTEN deficiency does not adversely affect either the thymic development or the function of Tregs, which retain their ability to suppress responder T cells in vitro and prevent colitis in vivo. Conversely, reexpression of PTEN in PTEN-deficient Tregs as well as in activated CD4+ T cells inhibits IL-2-dependent proliferation, confirming PTEN as a negative regulator of IL-2 receptor signaling. These data demonstrate that PTEN regulates the "anergic" response of Tregs to IL-2 in vitro and Treg homeostasis in vivo and indicate that inhibition of PTEN activity may facilitate the expansion of these cells for potential use in cellular immunotherapy.
Figures








References
-
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. - PubMed
-
- Mottet C., Uhlig H.H., Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 2003;170:3939–3943. - PubMed
-
- Taylor P.A., Lees C.J., Blazar B.R. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. - PubMed
-
- Takahashi T., et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

