Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M
- PMID: 16917541
- PMCID: PMC1550278
- DOI: 10.1172/JCI28054
Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M
Abstract
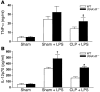
Sepsis results in a state of relative immunosuppression, rendering critically ill patients susceptible to secondary infections and increased mortality. Monocytes isolated from septic patients and experimental animals display a "deactivated" phenotype, characterized by impaired inflammatory and antimicrobial responses, including hyporesponsiveness to LPS. We investigated the role of the LPS/TLR4 axis and its inhibitor, IL-1 receptor-associated kinase-M (IRAK-M), in modulating the immunosuppression of sepsis using a murine model of peritonitis-induced sepsis followed by secondary challenge by intratracheal Pseudomonasaeruginosa. Septic mice demonstrated impaired alveolar macrophage function and increased mortality when challenged with intratracheal Pseudomonas as compared with nonseptic controls. TLR2 and TLR4 expression was unchanged in the lung following sepsis, whereas levels of IRAK-M were upregulated. Macrophages from IRAK-M-deficient septic mice produced higher levels of proinflammatory cytokines ex vivo and greater costimulatory molecule expression in vivo as compared with those of their WT counterparts. Following sepsis and secondary intrapulmonary bacterial challenge, IRAK-M(-/-) animals had higher survival rates and improved bacterial clearance from lung and blood compared with WT mice. In addition, increased pulmonary chemokine and inflammatory cytokine production was observed in IRAK-M(-/-) animals, leading to enhanced neutrophil recruitment to airspaces. Collectively, these findings indicate that IRAK-M mediates critical aspects of innate immunity that result in an immunocompromised state during sepsis.
Figures
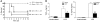

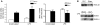

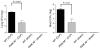


References
-
- Jacobs R.F., Kiel D.P., Balk R.A. Alveolar macrophage function in a canine model of endotoxin-induced lung injury. Am. Rev. Respir. Dis. 1986;134:745–751. - PubMed
-
- Nelson S., Chidiac C., Bagby G., Summer W.R. Endotoxin-induced suppression of lung host defenses. J. Med. 1990;21:85–103. - PubMed
-
- Volk H.D., et al. Monocyte deactivation — rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996;22(Suppl. 4):S474–S481. - PubMed
-
- Volk, H.D., et al. 1993. Alterations in function and phenotype of monocytes from patients with septic disease: predictive value and new therapeutic strategies. In Host defense dysfunction in trauma, shock, and sepsis. E. Faist, J.L. Meakins, and F.W. Schildberg, editors. Springer–Verlag. Berlin/Heidelberg, Germany. 365–371.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

