Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism
- PMID: 16917939
- PMCID: PMC2610366
- DOI: 10.1002/ajmg.b.30366
Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism
Abstract
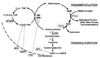
Autism is a behaviorally defined neurodevelopmental disorder usually diagnosed in early childhood that is characterized by impairment in reciprocal communication and speech, repetitive behaviors, and social withdrawal. Although both genetic and environmental factors are thought to be involved, none have been reproducibly identified. The metabolic phenotype of an individual reflects the influence of endogenous and exogenous factors on genotype. As such, it provides a window through which the interactive impact of genes and environment may be viewed and relevant susceptibility factors identified. Although abnormal methionine metabolism has been associated with other neurologic disorders, these pathways and related polymorphisms have not been evaluated in autistic children. Plasma levels of metabolites in methionine transmethylation and transsulfuration pathways were measured in 80 autistic and 73 control children. In addition, common polymorphic variants known to modulate these metabolic pathways were evaluated in 360 autistic children and 205 controls. The metabolic results indicated that plasma methionine and the ratio of S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH), an indicator of methylation capacity, were significantly decreased in the autistic children relative to age-matched controls. In addition, plasma levels of cysteine, glutathione, and the ratio of reduced to oxidized glutathione, an indication of antioxidant capacity and redox homeostasis, were significantly decreased. Differences in allele frequency and/or significant gene-gene interactions were found for relevant genes encoding the reduced folate carrier (RFC 80G > A), transcobalamin II (TCN2 776G > C), catechol-O-methyltransferase (COMT 472G > A), methylenetetrahydrofolate reductase (MTHFR 677C > T and 1298A > C), and glutathione-S-transferase (GST M1). We propose that an increased vulnerability to oxidative stress (endogenous or environmental) may contribute to the development and clinical manifestations of autism.
(c) 2006 Wiley-Liss, Inc.
Figures
References
-
- Afman LA, Lievers KJ, Van der Put NM, Trijbels FJ, Blom HJ. Single nucleotide polymorphisms in the transcobalamin gene: relationship with transcobalamin concentrations and risk for neural tube defects. Eur J Hum Genet. 2002;10:433–438. - PubMed
-
- Afman LA, Van der Put NMJ, Thomas CMG, Trijbels JMF, Blom HJ. Reduced vitamin B12 binding by transcobalamin II increases the risk of neural tube defects. Q J Med. 2001;94:159–166. - PubMed
-
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. - PubMed
-
- Bailey LB, Gregory JF., III Polymorphisms of methylenetetrahydrofolate reductase and other enzymes; metabolic significance, risks and impact on folate requirement. J Nutr , Supplement: Recent Advances in Nutritional Sciences. 2000;130:919–922. - PubMed
-
- Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 1997;25:335–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

