The human renal lymphatics under normal and pathological conditions
- PMID: 16918973
- PMCID: PMC1619848
- DOI: 10.1111/j.1365-2559.2006.02478.x
The human renal lymphatics under normal and pathological conditions
Abstract
Aims: The renal lymphatics have not been fully documented in humans. The aim of this study was to clarify the morphology of the human renal lymphatic system under normal and pathological conditions by immunohistochemistry using anti-D2-40 antibody.
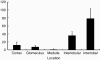
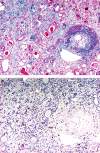
Methods and results: Normal and pathological renal tissues obtained at autopsy as well as nephrectomy specimens with renal cell carcinoma (RCC) were used. Thin sections were immunostained with antibodies against D2-40 and CD31. In normal kidney, D2-40+ lymphatics were abundant in the interstitium around the interlobar and arcuate arteries/veins but sporadic in those around the glomeruli or between the tubules in the cortex. A few lymphatics contained erythrocytes in their lumina. Lymphatics were seldom present in the medulla. In RCC cases, lymphatics were evident at the tumour margin, whereas CD31+ capillaries were abundant throughout the tumour and lymphatics were increased in the fibrous interstitium around the tumour. Lymphatic invasion by RCC cells was also detectable. D2-40+ lymphatics were evident in other pathological conditions and end-stage kidney had a denser lymphatic distribution than normal kidney.
Conclusions: Lymphatics are abundant around the arteries/veins and are also present in the renal cortex and medulla. D2-40 immunostaining is helpful for investigating the pathophysiological role of renal lymphatics.
Figures





References
-
- Rohn DA, Stewart RH, Elk JR, Laine GA, Drake RE. Renal lymphatic function following venous pressure elevation. Lymphology. 1996;29:67–75. - PubMed
-
- Holmes MJ, O'Morchoe PJ, O'Morchoe CCC. Morphology of the intrarenal lymphatic system; capsular and hilar communications. Am. J. Anat. 1977;149:333–352. - PubMed
-
- Cuttino JT, Jr, Jennette JC, Clark RL, Kwock L. Renal medullary lymphatics: microradiographic, light, and electron microscopic studies in pigs. Lymphology. 1985;18:24–30. - PubMed
-
- Cuttino JT, Jr, Clark RL, Jennette JC. Microradiographic demonstration of human intrarenal microlymphatic pathways. Urol. Radiol. 1989;11:83–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

