Mechanisms of major histocompatibility complex class II-restricted processing and presentation of the V antigen of Yersinia pestis
- PMID: 16919002
- PMCID: PMC1819574
- DOI: 10.1111/j.1365-2567.2006.02447.x
Mechanisms of major histocompatibility complex class II-restricted processing and presentation of the V antigen of Yersinia pestis
Abstract
We mapped mouse CD4 T-cell epitopes located in three structurally distinct regions of the V antigen of Yersinia pestis. T-cell hybridomas specific for epitopes from each region were generated to study the mechanisms of processing and presentation of V antigen by bone-marrow-derived macrophages. All three epitopes required uptake and/or processing from V antigen as well as presentation to T cells by newly synthesized major histocompatibility complex (MHC) class II molecules over a time period of 3-4 hr. Sensitivity to inhibitors showed a dependence on low pH and cysteine, serine and metalloproteinase, but not aspartic proteinase, activity. The data indicate that immunodominant epitopes from all three structural regions of V antigen were presented preferentially by the classical MHC class II-restricted presentation pathway. The requirement for processing by the co-ordinated activity of several enzyme families is consistent with the buried location of the epitopes in each region of V antigen. Understanding the structure-function relationship of multiple immunodominant epitopes of candidate subunit vaccines is necessary to inform choice of adjuvants for vaccine delivery. In the case of V antigen, adjuvants designed to target it to lysosomes are likely to induce optimal responses to multiple protective T-cell epitopes.
Figures

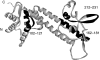

References
-
- Adamovicz JJ, Andrews GP. Plague vaccines. In: Lindler LE, Lebeda FJ, Korch LE, editors. Biological Weapons Defense. Totowa, NJ: Humana Press; 2005. pp. 121–53.
-
- Gage KL. Plague. In: Hausler WJ, Sussman M, editors. Topley and Wilson’s Microbiology and Microbial Infections. 9. Vol. 3. London: Arnold; 1998. pp. 885–903. chapter 44.
-
- Smego RA, Frean J, Koornhof HJ. Yersiniosis I. Microbiological and clinicoepidemiological aspects of plague and non-plague Yersinia infections. Eur J Clin Microbiol Infect Dis. 1999;18:1–15. - PubMed
-
- Cornelis GR. The Yersinia Ysc-Yop type III weaponry. Nat Rev Mol Cell Biol. 2002;3:742–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

