Immunogenicity and protection efficacy of monovalent and polyvalent poxvirus vaccines that include the D8 antigen
- PMID: 16919703
- PMCID: PMC7126721
- DOI: 10.1016/j.virol.2006.07.017
Immunogenicity and protection efficacy of monovalent and polyvalent poxvirus vaccines that include the D8 antigen
Abstract
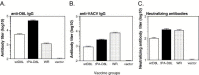
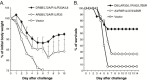
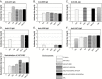
Recent studies have established the feasibility of subunit-based experimental vaccines to protect animals from lethal poxvirus infection. Individual outer membrane proteins from intracellular and extracellular virions of vaccinia virus, when delivered in the form of either DNA vaccines or recombinant protein vaccines produced from baculovirus-infected insect cells, were able to protect mice from the vaccinia virus challenge and rhesus macaques from the monkeypox virus challenge. The polyvalent formulations with various combinations of the four poxvirus antigens (A27, L1, B5 and A33) achieved better protection than the monovalent formulation using only one of these antigens. However, it is not clear whether any of the remaining outer membrane poxvirus proteins can further improve the efficacy of the current polyvalent formulations. In this study, we conducted detailed analysis on the immunogenicity of D8, a previously reported protective antigen from intracellular mature virions. Our results indicated that D8 induced strong protective antibody responses and was effective in improving the efficacy of previously reported polyvalent poxvirus vaccine formulations. Therefore, D8 is an excellent candidate antigen to be included in the final polyvalent subunit-based poxvirus vaccines.
Figures


Similar articles
-
Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions.J Virol. 2004 Oct;78(19):10230-7. doi: 10.1128/JVI.78.19.10230-10237.2004. J Virol. 2004. PMID: 15367588 Free PMC article.
-
Polyclonal antibody cocktails generated using DNA vaccine technology protect in murine models of orthopoxvirus disease.Virol J. 2011 Sep 20;8:441. doi: 10.1186/1743-422X-8-441. Virol J. 2011. PMID: 21933385 Free PMC article.
-
Immunogenicity and protection efficacy of subunit-based smallpox vaccines using variola major antigens.Virology. 2008 Feb 5;371(1):98-107. doi: 10.1016/j.virol.2007.09.029. Epub 2007 Oct 24. Virology. 2008. PMID: 17950773 Free PMC article.
-
Reflections on the early development of poxvirus vectors.Vaccine. 2013 Sep 6;31(39):4220-2. doi: 10.1016/j.vaccine.2013.03.042. Epub 2013 Apr 10. Vaccine. 2013. PMID: 23583893 Free PMC article. Review.
-
Polyvalent AIDS vaccines.Curr HIV Res. 2010 Dec;8(8):622-9. doi: 10.2174/157016210794088290. Curr HIV Res. 2010. PMID: 21054250 Free PMC article. Review.
Cited by
-
Potential threat of human pathogenic orthopoxviruses to public health and control strategies.J Biosaf Biosecur. 2023 Mar;5(1):1-7. doi: 10.1016/j.jobb.2022.12.004. Epub 2023 Jan 4. J Biosaf Biosecur. 2023. PMID: 36624850 Free PMC article. Review.
-
Side-by-side comparison of gene-based smallpox vaccine with MVA in nonhuman primates.PLoS One. 2012;7(7):e42353. doi: 10.1371/journal.pone.0042353. Epub 2012 Jul 31. PLoS One. 2012. PMID: 22860117 Free PMC article.
-
An epitope conserved in orthopoxvirus A13 envelope protein is the target of neutralizing and protective antibodies.Virology. 2011 Sep 15;418(1):67-73. doi: 10.1016/j.virol.2011.06.029. Epub 2011 Aug 2. Virology. 2011. PMID: 21810533 Free PMC article.
-
Functional epitopes and neutralizing antibodies of vaccinia virus.Front Microbiol. 2023 Oct 25;14:1255935. doi: 10.3389/fmicb.2023.1255935. eCollection 2023. Front Microbiol. 2023. PMID: 37954238 Free PMC article. Review.
-
Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine.J Virol. 2008 Apr;82(7):3751-68. doi: 10.1128/JVI.02244-07. Epub 2008 Jan 30. J Virol. 2008. PMID: 18234801 Free PMC article.
References
-
- Appleyard G., Hapel A.J., Boulter E.A. An antigenic difference between intracellular and extracellular rabbitpox virus. J. Gen. Virol. 1971;13(1):9–17. - PubMed
-
- Artenstein A.W., Johnson C., Marbury T.C., Morrison D., Blum P.S., Kemp T., Nichols R., Balser J.P., Currie M., Monath T.P. A novel, cell culture-derived smallpox vaccine in vaccinia-naive adults. Vaccine. 2005;23(25):3301–3309. - PubMed
-
- Boulter E.A., Appleyard G. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog. Med. Virol. 1973;16:86–108. - PubMed
-
- Chen H.D., Fraire A.E., Joris I., Brehm M.A., Welsh R.M., Selin L.K. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat. Immunol. 2001;2(11):1067–1076. - PubMed
-
- Chernos V.I., Vovk T.S., Ivanova O.N., Antonova T.P., Loparev V.N. Insertion mutants of the vaccinia virus. The effect of inactivating E7R and D8L genes on the biological properties of the virus. Mol. Gen. Mikrobiol. Virusol. 1993;(2):30–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

