Optical and pharmacological tools to investigate the role of mitochondria during oxidative stress and neurodegeneration
- PMID: 16920246
- PMCID: PMC1994087
- DOI: 10.1016/j.pneurobio.2006.07.001
Optical and pharmacological tools to investigate the role of mitochondria during oxidative stress and neurodegeneration
Abstract
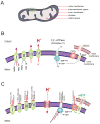
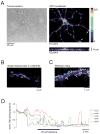
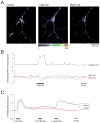
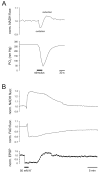
Mitochondria are critical for cellular adenosine triphosphate (ATP) production; however, recent studies suggest that these organelles fulfill a much broader range of tasks. For example, they are involved in the regulation of cytosolic Ca(2+) levels, intracellular pH and apoptosis, and are the major source of reactive oxygen species (ROS). Various reactive molecules that originate from mitochondria, such as ROS, are critical in pathological events, such as ischemia, as well as in physiological events such as long-term potentiation, neuronal-vascular coupling and neuronal-glial interactions. Due to their key roles in the regulation of several cellular functions, the dysfunction of mitochondria may be critical in various brain disorders. There has been increasing interest in the development of tools that modulate mitochondrial function, and the refinement of techniques that allow for real time monitoring of mitochondria, particularly within their intact cellular environment. Innovative imaging techniques are especially powerful since they allow for mitochondrial visualization at high resolution, tracking of mitochondrial structures and optical real time monitoring of parameters of mitochondrial function. The techniques discussed include classic imaging techniques, such as rhodamine-123, the highly advanced semi-conductor nanoparticles (quantum dots), and wide field microscopy as well as high-resolution multiphoton imaging. We have highlighted the use of these techniques to study mitochondrial function in brain tissue and have included studies from our laboratories in which these techniques have been successfully applied.
Figures
Similar articles
-
Alternative mitochondrial functions in cell physiopathology: beyond ATP production.Braz J Med Biol Res. 2000 Feb;33(2):241-50. doi: 10.1590/s0100-879x2000000200014. Braz J Med Biol Res. 2000. PMID: 10657067 Review.
-
Oxidative stress: emerging mitochondrial and cellular themes and variations in neuronal injury.J Alzheimers Dis. 2010;20 Suppl 2:S453-73. doi: 10.3233/JAD-2010-100321. J Alzheimers Dis. 2010. PMID: 20463398 Review.
-
Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes.J Pineal Res. 2004 Aug;37(1):55-70. doi: 10.1111/j.1600-079X.2004.00140.x. J Pineal Res. 2004. PMID: 15230869
-
Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death.Plant Physiol. 2002 Apr;128(4):1271-81. doi: 10.1104/pp.010999. Plant Physiol. 2002. PMID: 11950976 Free PMC article.
-
Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction.J Neurochem. 2008 Oct;107(1):265-78. doi: 10.1111/j.1471-4159.2008.05616.x. Epub 2008 Aug 9. J Neurochem. 2008. PMID: 18691386
Cited by
-
Temporo-spectral imaging of intrinsic optical signals during hypoxia-induced spreading depression-like depolarization.PLoS One. 2012;7(8):e43981. doi: 10.1371/journal.pone.0043981. Epub 2012 Aug 29. PLoS One. 2012. PMID: 22952835 Free PMC article.
-
Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa.Int J Nanomedicine. 2012;7:5901-14. doi: 10.2147/IJN.S37397. Epub 2012 Nov 30. Int J Nanomedicine. 2012. PMID: 23226696 Free PMC article.
-
Febuxostat pretreatment attenuates myocardial ischemia/reperfusion injury via mitochondrial apoptosis.J Transl Med. 2015 Jul 2;13:209. doi: 10.1186/s12967-015-0578-x. J Transl Med. 2015. PMID: 26136232 Free PMC article.
-
Detailed analysis of apoptosis and delayed luminescence of human leukemia Jurkat T cells after proton irradiation and treatments with oxidant agents and flavonoids.Oxid Med Cell Longev. 2012;2012:498914. doi: 10.1155/2012/498914. Epub 2012 Jul 3. Oxid Med Cell Longev. 2012. PMID: 22829956 Free PMC article.
-
Mitochondrial viability in mouse and human postmortem brain.FASEB J. 2010 Sep;24(9):3590-9. doi: 10.1096/fj.09-152108. Epub 2010 May 13. FASEB J. 2010. PMID: 20466876 Free PMC article.
References
-
- Albracht SP, van der Linden E, Faber BW. Quantitative amino acid analysis of bovine NADH: ubiquinone oxidoreductase (Complex I) and related enzymes. Consequences for the number of prosthetic groups. Biochim Biophys Acta. 2003;1557:41–9. - PubMed
-
- Almeida A, Bolanos JP. A transient inhibition of mitochondrial ATP synthesis by nitric oxide synthase activation triggered apoptosis in primary cortical neurons. J Neurochem. 2001;77:676–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

