Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum
- PMID: 16920778
- PMCID: PMC1560902
- DOI: 10.1105/tpc.106.043695
Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum
Abstract
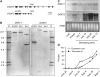
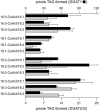
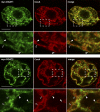
Seeds of the tung tree (Vernicia fordii) produce large quantities of triacylglycerols (TAGs) containing approximately 80% eleostearic acid, an unusual conjugated fatty acid. We present a comparative analysis of the genetic, functional, and cellular properties of tung type 1 and type 2 diacylglycerol acyltransferases (DGAT1 and DGAT2), two unrelated enzymes that catalyze the committed step in TAG biosynthesis. We show that both enzymes are encoded by single genes and that DGAT1 is expressed at similar levels in various organs, whereas DGAT2 is strongly induced in developing seeds at the onset of oil biosynthesis. Expression of DGAT1 and DGAT2 in yeast produced different types and proportions of TAGs containing eleostearic acid, with DGAT2 possessing an enhanced propensity for the synthesis of trieleostearin, the main component of tung oil. Both DGAT1 and DGAT2 are located in distinct, dynamic regions of the endoplasmic reticulum (ER), and surprisingly, these regions do not overlap. Furthermore, although both DGAT1 and DGAT2 contain a similar C-terminal pentapeptide ER retrieval motif, this motif alone is not sufficient for their localization to specific regions of the ER. These data suggest that DGAT1 and DGAT2 have nonredundant functions in plants and that the production of storage oils, including those containing unusual fatty acids, occurs in distinct ER subdomains.
Figures
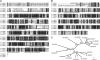





References
-
- Bischoff, F., Vahlkamp, L., Molendijk, A., and Palme, K. (2000). Localization of AtROP4 and AtROP6 and interaction with the guanine nucleotide dissociation inhibitor AtRhoGDI1 from Arabidopsis. Plant Mol. Biol. 42 515–530. - PubMed
-
- Bland, J.M., Park, Y.I., Raina, A.K., Dickens, J.C., and Hollister, B. (2004). Trilinolein identified as a sex-specific component of tergal glands in alates of Coptotermes formosanus. J. Chem. Ecol. 30 835–849. - PubMed
-
- Bligh, E.G., and Dyer, W.J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37 911–917. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

