Single-molecule mechanics of mussel adhesion
- PMID: 16920796
- PMCID: PMC1559742
- DOI: 10.1073/pnas.0605552103
Single-molecule mechanics of mussel adhesion
Abstract
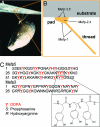
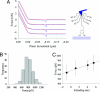
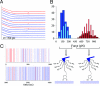
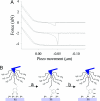
The glue proteins secreted by marine mussels bind strongly to virtually all inorganic and organic surfaces in aqueous environments in which most adhesives function poorly. Studies of these functionally unique proteins have revealed the presence of the unusual amino acid 3,4-dihydroxy-L-phenylalanine (dopa), which is formed by posttranslational modification of tyrosine. However, the detailed binding mechanisms of dopa remain unknown, and the chemical basis for mussels' ability to adhere to both inorganic and organic surfaces has never been fully explained. Herein, we report a single-molecule study of the substrate and oxidation-dependent adhesive properties of dopa. Atomic force microscopy (AFM) measurements of a single dopa residue contacting a wet metal oxide surface reveal a surprisingly high strength yet fully reversible, noncovalent interaction. The magnitude of the bond dissociation energy as well as the inability to observe this interaction with tyrosine suggests that dopa is critical to adhesion and that the binding mechanism is not hydrogen bond formation. Oxidation of dopa, as occurs during curing of the secreted mussel glue, dramatically reduces the strength of the interaction to metal oxide but results in high strength irreversible covalent bond formation to an organic surface. A new picture of the interfacial adhesive role of dopa emerges from these studies, in which dopa exploits a remarkable combination of high strength and chemical multifunctionality to accomplish adhesion to substrates of widely varying composition from organic to metallic.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
References
-
- Autumn K., Liang Y. A., Hsieh S. T., Zesch W., Chan W. P., Kenny T. W., Fearing R., Full R. J. Nature. 2000;405:681–685. - PubMed
-
- Chisholm J. R. M., Kelley R. Nature. 2001;409:152. - PubMed
-
- Waite J. H. Integr. Comp. Biol. 2002;42:1172–1180. - PubMed
-
- Waite J. H., Tanzer M. L. Science. 1981;212:1038–1040. - PubMed
-
- Crisp D. J., Walker G., Young G. A., Yule A. B. J. Colloid Interface Sci. 1985;104:40–50.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

