Putative cis-acting stem-loops in the 5' untranslated region of the severe acute respiratory syndrome coronavirus can substitute for their mouse hepatitis virus counterparts
- PMID: 16920822
- PMCID: PMC1641749
- DOI: 10.1128/JVI.00455-06
Putative cis-acting stem-loops in the 5' untranslated region of the severe acute respiratory syndrome coronavirus can substitute for their mouse hepatitis virus counterparts
Abstract
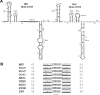
Consensus covariation-based secondary structural models for the 5' 140 nucleotides of the 5' untranslated regions (5'UTRs) from mouse hepatitis virus (MHV) and severe acute respiratory syndrome coronavirus (SCoV) were developed and predicted three major helical stem-loop structures, designated stem-loop 1 (SL1), SL2, and SL4. The SCoV 5'UTR was predicted to contain a fourth stem-loop, named SL3, in which the leader transcriptional regulatory sequence (TRS) is folded into a hairpin loop. cDNAs corresponding to MHV/SCoV chimeric genomes were constructed by replacing the complete MHV 5'UTR with the corresponding SCoV sequence and by separately replacing MHV 5'UTR putative SL1, putative SL2, TRS, and putative SL4 with the corresponding SCoV sequences. Chimeric genomes were transcribed in vitro, and viruses were recovered after electroporation into permissive cells. Genomes in which the MHV 5'UTR SL1, SL2, and SL4 were individually replaced by their SCoV counterparts were viable. Chimeras containing the complete SCoV 5'UTR or the predicted SCoV SL3 were not viable. A chimera containing the SCoV 5'UTR in which the SCoV TRS was replaced with the MHV TRS was also not viable. The chimera containing the entire SCoV 5'UTR failed to direct the synthesis of any virus-specific RNA. Replacing the SCoV TRS with the MHV TRS in the MHV/5'UTR SCoV chimera permitted the synthesis of minus-sense genome-sized RNA but did not support the production of positive- or minus-sense subgenomic RNA7. A similar phenotype was obtained with the MHV/SCoV SL3 chimera. These results suggest a role for the TRS in the replication of minus-sense genomic RNA in addition to its known function in subgenomic RNA synthesis.
Figures




Similar articles
-
Mouse hepatitis virus stem-loop 4 functions as a spacer element required to drive subgenomic RNA synthesis.J Virol. 2011 Sep;85(17):9199-209. doi: 10.1128/JVI.05092-11. Epub 2011 Jun 29. J Virol. 2011. PMID: 21715502 Free PMC article.
-
A U-turn motif-containing stem-loop in the coronavirus 5' untranslated region plays a functional role in replication.RNA. 2007 May;13(5):763-80. doi: 10.1261/rna.261807. Epub 2007 Mar 12. RNA. 2007. PMID: 17353353 Free PMC article.
-
Promoter activity of SARS coronavirus 5' UTR sequence in eukaryotic cells.Sichuan Da Xue Xue Bao Yi Xue Ban. 2006 Jan;37(1):5-9. Sichuan Da Xue Xue Bao Yi Xue Ban. 2006. PMID: 16468630
-
The structure and functions of coronavirus genomic 3' and 5' ends.Virus Res. 2015 Aug 3;206:120-33. doi: 10.1016/j.virusres.2015.02.025. Epub 2015 Feb 28. Virus Res. 2015. PMID: 25736566 Free PMC article. Review.
-
Development of mouse hepatitis virus and SARS-CoV infectious cDNA constructs.Curr Top Microbiol Immunol. 2005;287:229-52. doi: 10.1007/3-540-26765-4_8. Curr Top Microbiol Immunol. 2005. PMID: 15609514 Free PMC article. Review.
Cited by
-
Classification, replication, and transcription of Nidovirales.Front Microbiol. 2024 Jan 24;14:1291761. doi: 10.3389/fmicb.2023.1291761. eCollection 2023. Front Microbiol. 2024. PMID: 38328580 Free PMC article. Review.
-
A 5'-proximal stem-loop structure of 5' untranslated region of porcine reproductive and respiratory syndrome virus genome is key for virus replication.Virol J. 2011 Apr 15;8:172. doi: 10.1186/1743-422X-8-172. Virol J. 2011. PMID: 21496223 Free PMC article.
-
RNA structure analysis of alphacoronavirus terminal genome regions.Virus Res. 2014 Dec 19;194:76-89. doi: 10.1016/j.virusres.2014.10.001. Epub 2014 Oct 13. Virus Res. 2014. PMID: 25307890 Free PMC article. Review.
-
Continuous and Discontinuous RNA Synthesis in Coronaviruses.Annu Rev Virol. 2015 Nov;2(1):265-88. doi: 10.1146/annurev-virology-100114-055218. Annu Rev Virol. 2015. PMID: 26958916 Free PMC article. Review.
-
An evolutionarily conserved strategy for ribosome binding and inhibition by β-coronavirus non-structural protein 1.bioRxiv [Preprint]. 2023 Jun 8:2023.06.07.544141. doi: 10.1101/2023.06.07.544141. bioRxiv. 2023. Update in: J Mol Biol. 2023 Oct 15;435(20):168259. doi: 10.1016/j.jmb.2023.168259. PMID: 37333070 Free PMC article. Updated. Preprint.
References
-
- Bredenbeek, P. J., C. J. Pachuk, A. F. Noten, J. Charite, W. Luytjes, S. R. Weiss, and W. J. Spaan. 1990. The primary structure and expression of the second open reading frame of the polymerase gene of the coronavirus MHV-A59; a highly conserved polymerase is expressed by an efficient ribosomal frameshifting mechanism. Nucleic Acids Res. 18:1825-1832. - PMC - PubMed
-
- Campbell, D. O., and P. Legault. 2005. Nuclear magnetic resonance structure of the Varkud satellite ribozyme stem-loop V RNA and magnesium-ion binding from chemical-shift mapping. Biochemistry 44:4157-4170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

