Regulation of Mycobacterium tuberculosis whiB3 in the mouse lung and macrophages
- PMID: 16923787
- PMCID: PMC1695489
- DOI: 10.1128/IAI.00190-06
Regulation of Mycobacterium tuberculosis whiB3 in the mouse lung and macrophages
Abstract
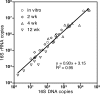
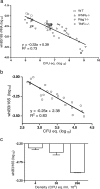
Mycobacterium tuberculosis is a highly successful human pathogen, with approximately 2x10(9) individuals infected globally. To understand the responses of M. tuberculosis to the in vivo environment, we studied the in vivo regulation of M. tuberculosis genes whose M. marinum homologs are induced in chronically infected frog tissues. The expression of 16S rRNA was shown to remain constant in M. tuberculosis under in vivo and in vitro conditions and therefore could be used for internal normalization in quantitative reverse transcription-PCR assays. We found whiB3, a putative transcriptional regulator implicated in mediating tissue damage, to be maximally induced at 2 weeks postinfection in the lungs of wild-type and immunodeficient (gamma interferon receptor-/-, Rag1-/-, and tumor necrosis factor alpha-/-) mice. At later time points in wild-type mice, whiB3 induction was decreased and gradually declined over the course of infection. In immunodeficient mice, whiB3 induction declined rapidly and was completely abolished in moribund animals. whiB3 was also found to be induced in naïve bone marrow-derived macrophages after 6 h of infection. whiB3 expression in vivo and in vitro was found to be inversely correlated with bacterial density. These results indicate that M. tuberculosis regulates the expression of whiB3 in response to environmental signals present in vivo and are consistent with a model of regulation by quorum sensing.
Figures
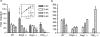
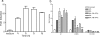

Similar articles
-
PhoPR Positively Regulates whiB3 Expression in Response to Low pH in Pathogenic Mycobacteria.J Bacteriol. 2018 Mar 26;200(8):e00766-17. doi: 10.1128/JB.00766-17. Print 2018 Apr 15. J Bacteriol. 2018. PMID: 29378889 Free PMC article.
-
Mycobacterium tuberculosis WhiB3 interacts with RpoV to affect host survival but is dispensable for in vivo growth.Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):3147-52. doi: 10.1073/pnas.052705399. Proc Natl Acad Sci U S A. 2002. PMID: 11880648 Free PMC article.
-
Mycobacterium tuberculosis arrests host cycle at the G1/S transition to establish long term infection.PLoS Pathog. 2017 May 22;13(5):e1006389. doi: 10.1371/journal.ppat.1006389. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28542477 Free PMC article.
-
The AraC family transcriptional regulator Rv1931c plays a role in the virulence of Mycobacterium tuberculosis.Infect Immun. 2004 Sep;72(9):5483-6. doi: 10.1128/IAI.72.9.5483-5486.2004. Infect Immun. 2004. PMID: 15322050 Free PMC article.
-
Extra and intracellular expression of Mycobacterium tuberculosis genes.Tuber Lung Dis. 1998;79(2):91-7. doi: 10.1054/tuld.1998.0010. Tuber Lung Dis. 1998. PMID: 10645446 Review.
Cited by
-
Quorum Sensing: An Under-Explored Phenomenon in the Phylum Actinobacteria.Front Microbiol. 2016 Feb 10;7:131. doi: 10.3389/fmicb.2016.00131. eCollection 2016. Front Microbiol. 2016. PMID: 26904007 Free PMC article. Review.
-
Host cell-induced components of the sulfate assimilation pathway are major protective antigens of Mycobacterium tuberculosis.J Infect Dis. 2013 Mar 1;207(5):778-85. doi: 10.1093/infdis/jis751. Epub 2012 Dec 7. J Infect Dis. 2013. PMID: 23225904 Free PMC article.
-
A walk into the LuxR regulators of Actinobacteria: phylogenomic distribution and functional diversity.PLoS One. 2012;7(10):e46758. doi: 10.1371/journal.pone.0046758. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056438 Free PMC article.
-
Coincidence cloning recovery of Brucella melitensis RNA from goat tissues: advancing the in vivo analysis of pathogen gene expression in brucellosis.BMC Mol Biol. 2018 Aug 1;19(1):10. doi: 10.1186/s12867-018-0111-x. BMC Mol Biol. 2018. PMID: 30068312 Free PMC article.
-
LprG-mediated surface expression of lipoarabinomannan is essential for virulence of Mycobacterium tuberculosis.PLoS Pathog. 2014 Sep 18;10(9):e1004376. doi: 10.1371/journal.ppat.1004376. eCollection 2014 Sep. PLoS Pathog. 2014. PMID: 25232742 Free PMC article.
References
-
- Bardarov, S., S. Bardarov, Jr., M. S. Pavelka, Jr., V. Sambandamurthy, M. Larsen, J. Tufariello, J. Chan, G. Hatfull, and W. R. Jacobs, Jr. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148:3007-3017. - PubMed
-
- Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. - PubMed
-
- Dahl, J. L., C. N. Kraus, H. I. Boshoff, B. Doan, K. Foley, D. Avarbock, G. Kaplan, V. Mizrahi, H. Rubin, and C. E. Barry III. 2003. The role of M. tuberculosis-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc. Natl. Acad. Sci. USA 100:10026-10031. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

