Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction
- PMID: 16923853
- PMCID: PMC2118387
- DOI: 10.1084/jem.20061022
Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction
Abstract
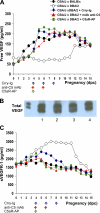
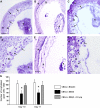
Immune mechanisms have been implicated in placental dysfunction in patients with recurrent miscarriages and intrauterine growth restriction (IUGR), but the mediators are undefined. Here we show that complement activation, particularly C5a, is a required intermediary event in the pathogenesis of placental and fetal injury in an antibody-independent mouse model of spontaneous miscarriage and IUGR, and that complement activation causes dysregulation of the angiogenic factors required for normal placental development. Pregnancies complicated by miscarriage or growth restriction were characterized by inflammatory infiltrates in placentas, functional deficiency of free vascular endothelial growth factor (VEGF), elevated levels of soluble VEGF receptor 1 (sVEGFR-1, also known as sFlt-1; a potent anti-angiogenic molecule), and defective placental development. Inhibition of complement activation in vivo blocked the increase in sVEGFR-1 and rescued pregnancies. In vitro stimulation of monocytes with products of the complement cascade directly triggered release of sVEGFR-1, which sequesters VEGF. These studies provide the first evidence linking the complement system to angiogenic factor imbalance associated with placental dysfunction, and identify a new effector of immune-triggered pregnancy complications.
Figures


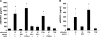
References
-
- Mills, J.L., J.L. Simpson, S.G. Driscoll, L. Jovanovic-Peterson, M. Van Allen, J.H. Aarons, B. Metzger, F.R. Bieber, R.H. Knopp, L.B. Holmes, et al. 1988. Incidence of spontaneous abortion among normal women and insulin-dependent diabetic women whose pregnancies were identified within 21 days of conception. N. Engl. J. Med. 319:1617–1623. - PubMed
-
- Resnik, R., and R.K. Creasy. 2003. Intrauterine growth restriction. In Maternal-Fetal Medicine: Principles and Practice. J.D. Iams, editor. Saunders, Philadelphia. 495–512.
-
- Godfrey, K.M., and D.J. Barker. 2000. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 71:1344S–1352S. - PubMed
-
- Mellor, A.L., and D.H. Munn. 2000. Immunology at the maternal-fetal interface: lessons for T cell tolerance and suppression. Annu. Rev. Immunol. 18:367–391. - PubMed
-
- Hunt, J.S., M.G. Petroff, R.H. McIntire, and C. Ober. 2005. HLA-G and immune tolerance in pregnancy. FASEB J. 19:681–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

