Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix
- PMID: 16924102
- PMCID: PMC1551904
- DOI: 10.1073/pnas.0605669103
Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix
Abstract
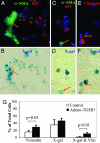
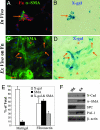
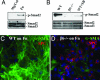
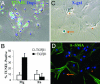
Mechanisms leading to fibroblast accumulation during pulmonary fibrogenesis remain unclear. Although there is in vitro evidence of lung alveolar epithelial-to-mesenchymal transition (EMT), whether EMT occurs within the lung is currently unknown. Biopsies from fibrotic human lungs demonstrate epithelial cells with mesenchymal features, suggesting EMT. To more definitively test the capacity of alveolar epithelial cells for EMT, mice expressing beta-galactosidase (beta-gal) exclusively in lung epithelial cells were generated, and their fates were followed in an established model of pulmonary fibrosis, overexpression of active TGF-beta1. beta-gal-positive cells expressing mesenchymal markers accumulated within 3 weeks of in vivo TGF-beta1 expression. The increase in vimentin-positive cells within injured lungs was nearly all beta-gal-positive, indicating epithelial cells as the main source of mesenchymal expansion in this model. Ex vivo, primary alveolar epithelial cells cultured on provisional matrix components, fibronectin or fibrin, undergo robust EMT via integrin-dependent activation of endogenous latent TGF-beta1. In contrast, primary cells cultured on laminin/collagen mixtures do not activate the TGF-beta1 pathway and, if exposed to active TGF-beta1, undergo apoptosis rather than EMT. These data reveal alveolar epithelial cells as progenitors for fibroblasts in vivo and implicate the provisional extracellular matrix as a key regulator of epithelial transdifferentiation during fibrogenesis.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

