Kelch-repeat proteins interacting with the Galpha protein Gpa2 bypass adenylate cyclase for direct regulation of protein kinase A in yeast
- PMID: 16924114
- PMCID: PMC1559748
- DOI: 10.1073/pnas.0509644103
Kelch-repeat proteins interacting with the Galpha protein Gpa2 bypass adenylate cyclase for direct regulation of protein kinase A in yeast
Abstract
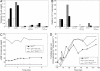
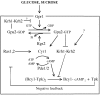
The cAMP-PKA pathway consists of an extracellular ligand-sensitive G protein-coupled receptor, a G protein signal transmitter, and the effector, adenylate cyclase, of which the product, cAMP, acts as an intracellular second messenger. cAMP activates PKA by dissociating the regulatory subunit from the catalytic subunit. Yeast cells (Saccharomyces cerevisiae) contain a glucose/sucrose-sensitive seven-transmembrane domain receptor, Gpr1, that was proposed to activate adenylate cyclase through the G(alpha) protein Gpa2. Consistently, we show here that adenylate cyclase binds only to active, GTP-bound Gpa2. Two related kelch-repeat proteins, Krh1/Gpb2 and Krh2/Gpb1, are associated with Gpa2 and were suggested to act as G(beta) mimics for Gpa2, based on their predicted seven-bladed beta-propeller structure. However, we find that although Krh1 associates with both GDP and GTP-bound Gpa2, it displays a preference for GTP-Gpa2. The strong down-regulation of PKA targets by Krh1 and Krh2 does not require Gpa2 but is strictly dependent on both the catalytic and the regulatory subunits of PKA. Krh1 directly interacts with PKA by means of the catalytic subunits, and Krh1/2 stimulate the association between the catalytic and regulatory subunits in vivo. Indeed, both a constitutively active GPA2 allele and deletion of KRH1/2 lower the cAMP requirement of PKA for growth. We propose that active Gpa2 relieves the inhibition imposed by the kelch-repeat proteins on PKA, thereby bypassing adenylate cyclase for direct regulation of PKA. Importantly, we show that Krh1/2 also enhance the association between mouse R and C subunits, suggesting that Krh control of PKA has been evolutionarily conserved.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
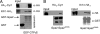

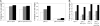

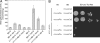
Similar articles
-
GPR1 encodes a putative G protein-coupled receptor that associates with the Gpa2p Galpha subunit and functions in a Ras-independent pathway.EMBO J. 1998 Apr 1;17(7):1996-2007. doi: 10.1093/emboj/17.7.1996. EMBO J. 1998. PMID: 9524122 Free PMC article.
-
Glucose-stimulated cAMP-protein kinase A pathway in yeast Saccharomyces cerevisiae.J Biosci Bioeng. 2007 Oct;104(4):245-50. doi: 10.1263/jbb.104.245. J Biosci Bioeng. 2007. PMID: 18023794 Review.
-
Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans.Eukaryot Cell. 2004 Aug;3(4):919-31. doi: 10.1128/EC.3.4.919-931.2004. Eukaryot Cell. 2004. PMID: 15302825 Free PMC article.
-
G-protein-coupled receptor Gpr1 and G-protein Gpa2 of cAMP-dependent signaling pathway are involved in glucose-induced pexophagy in the yeast Saccharomyces cerevisiae.Cell Biol Int. 2008 May;32(5):502-4. doi: 10.1016/j.cellbi.2007.11.001. Epub 2007 Nov 12. Cell Biol Int. 2008. PMID: 18096414
-
Directly from Galpha to protein kinase A: the kelch repeat protein bypass of adenylate cyclase.Trends Biochem Sci. 2007 Dec;32(12):547-54. doi: 10.1016/j.tibs.2007.09.011. Epub 2007 Nov 5. Trends Biochem Sci. 2007. PMID: 17983752 Review.
Cited by
-
A novel yeast hybrid modeling framework integrating Boolean and enzyme-constrained networks enables exploration of the interplay between signaling and metabolism.PLoS Comput Biol. 2021 Apr 9;17(4):e1008891. doi: 10.1371/journal.pcbi.1008891. eCollection 2021 Apr. PLoS Comput Biol. 2021. PMID: 33836000 Free PMC article.
-
D-Xylose Sensing in Saccharomyces cerevisiae: Insights from D-Glucose Signaling and Native D-Xylose Utilizers.Int J Mol Sci. 2021 Nov 17;22(22):12410. doi: 10.3390/ijms222212410. Int J Mol Sci. 2021. PMID: 34830296 Free PMC article. Review.
-
Yeast 3-phosphoinositide-dependent protein kinase-1 (PDK1) orthologs Pkh1-3 differentially regulate phosphorylation of protein kinase A (PKA) and the protein kinase B (PKB)/S6K ortholog Sch9.J Biol Chem. 2011 Jun 24;286(25):22017-27. doi: 10.1074/jbc.M110.200071. Epub 2011 Apr 29. J Biol Chem. 2011. PMID: 21531713 Free PMC article.
-
Multiple Transceptors for Macro- and Micro-Nutrients Control Diverse Cellular Properties Through the PKA Pathway in Yeast: A Paradigm for the Rapidly Expanding World of Eukaryotic Nutrient Transceptors Up to Those in Human Cells.Front Pharmacol. 2018 Mar 13;9:191. doi: 10.3389/fphar.2018.00191. eCollection 2018. Front Pharmacol. 2018. PMID: 29662449 Free PMC article. Review.
-
Saccharomyces cerevisiae phospholipase C regulates transcription of Msn2p-dependent stress-responsive genes.Eukaryot Cell. 2008 Jun;7(6):967-79. doi: 10.1128/EC.00438-07. Epub 2008 Mar 28. Eukaryot Cell. 2008. PMID: 18375619 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

