Soluble fibrin inhibits monocyte adherence and cytotoxicity against tumor cells: implications for cancer metastasis
- PMID: 16925817
- PMCID: PMC1564130
- DOI: 10.1186/1477-9560-4-12
Soluble fibrin inhibits monocyte adherence and cytotoxicity against tumor cells: implications for cancer metastasis
Abstract
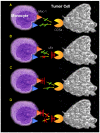
Background: Soluble fibrin (sFn) is a marker for disseminated intravascular coagulation and may have prognostic significance, especially in metastasis. However, a role for sFn in the etiology of metastatic cancer growth has not been extensively studied. We have reported that sFn cross-linked platelet binding to tumor cells via the major platelet fibrin receptor alphaIIb beta3, and tumor cell CD54 (ICAM-1), which is the receptor for two of the leukocyte beta2 integrins (alphaL beta2 and aM beta2). We hypothesized that sFn may also affect leukocyte adherence, recognition, and killing of tumor cells. Furthermore, in a rat experimental metastasis model sFn pre-treatment of tumor cells enhanced metastasis by over 60% compared to untreated cells. Other studies have shown that fibrin(ogen) binds to the monocyte integrin alphaM beta2. This study therefore sought to investigate the effect of sFn on beta2 integrin mediated monocyte adherence and killing of tumor cells.
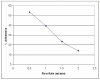
Methods: The role of sFn in monocyte adherence and cytotoxicity against tumor cells was initially studied using static microplate adherence and cytotoxicity assays, and under physiologically relevant flow conditions in a microscope perfusion incubator system. Blocking studies were performed using monoclonal antibodies specific for beta2 integrins and CD54, and specific peptides which inhibit sFn binding to these receptors.
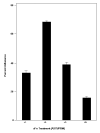
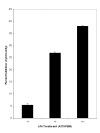
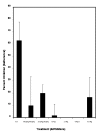
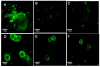
Results: Enhancement of monocyte/tumor cell adherence was observed when only one cell type was bound to sFn, but profound inhibition was observed when sFn was bound to both monocytes and tumor cells. This effect was also reflected in the pattern of monocyte cytotoxicity. Studies using monoclonal blocking antibodies and specific blocking peptides (which did not affect normal coagulation) showed that the predominant mechanism of fibrin inhibition is via its binding to alphaM beta2 on monocytes, and to CD54 on both leukocytes and tumor cells.
Conclusion: sFn inhibits monocyte adherence and cytotoxicity of tumor cells by blocking alphaL beta2 and alphaM beta2 binding to tumor cell CD54. These results demonstrate that sFn is immunosuppressive and may be directly involved in the etiology of metastasis. Use of specific peptides also inhibited this effect without affecting coagulation, suggesting their possible use as novel therapeutic agents in cancer metastasis.
Figures




References
-
- Trousseau A. Phlegmasia alba dolens. Clinique medicale de l'hotel de Paris. 1865;3
-
- Gouin-Thibault I, Samama MM. Laboratory diagnosis of the thrombophilic state in cancer patients. Semin Thromb Hemost. 1999;25:167–172. - PubMed
-
- Nakagawa K, Tsuji H, Masuda H, Yamada K, Yamada Y, Yoneda M. Plasma levels of soluble fibrin in patients with malignancy-associated disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 1994;5:725–730. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

