T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells
- PMID: 16926291
- PMCID: PMC1895462
- DOI: 10.1182/blood-2006-04-017061
T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells
Abstract
There has been interest in generating T cells expressing chimeric artificial receptors (CARs) targeting CD19/CD20 antigens to treat B-cell lymphomas. If successful, however, this approach would likely impair humoral immunity because T cells may persist long-term. Most low-grade lymphoma and chronic lymphocytic leukemia (B-CLL) cells express monoclonal immunoglobulins carrying either kappa or lambda light chains. We, therefore, explored whether T lymphocytes could be genetically modified to target the tumor-associated light chain, sparing B lymphocytes expressing the reciprocal light chain, and consequently reduce impairment of humoral immunity. We found that T lymphocytes expressing the anti-kappa light chain CAR showed cytotoxic activity against Igkappa(+) tumor cell lines and B-CLL cells both in vitro and in vivo. We also found that the incorporation of the CD28 endodomain within the CAR enhanced the in vitro and in vivo expansion of transgenic T cells after tumor-associated antigen stimulation. Free Igkappa(+) did not compromise the ability of redirected T lymphocytes to eliminate Igkappa(+) tumors because these free immunoglobulins served to sustain proliferation of CAR-CD28 transgenic T cells. Thus, adoptive transfer of T lymphocytes targeting the appropriate light chain could be a useful immunotherapy approach to treat B-lymphocyte malignancies that clonally express immunoglobulin without entirely compromising humoral immunity.
Figures

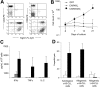
 ) or CAR46/28ζ (▪). Cytotoxic activity was evaluated in a standard 51Cr-release assay, and results are shown at an E/T ratio of 20:1. Data represent the mean ± SD of 6 different donors.
) or CAR46/28ζ (▪). Cytotoxic activity was evaluated in a standard 51Cr-release assay, and results are shown at an E/T ratio of 20:1. Data represent the mean ± SD of 6 different donors.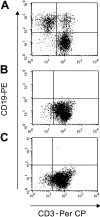
 ) or control T cells (□) after stimulation with autologous B-CLL cells. Data represent mean ± SD of 4 different donors. The specificity of expanded T lymphocytes was then evaluated using a CFSE-based cytotoxicity assay (E/T ratio, 20:1). Panel D illustrates that CAR46/28ζ T lymphocytes (▪) killed autologous and allogeneic κ+ B-CLL cells, but not allogeneic κ+ B-CLL cells. In contrast, no significant killing was observed for control transduced T lymphocytes expanded in the presence of exogenous IL-2 (□). Data represent mean ± SD of 3 different donors.
) or control T cells (□) after stimulation with autologous B-CLL cells. Data represent mean ± SD of 4 different donors. The specificity of expanded T lymphocytes was then evaluated using a CFSE-based cytotoxicity assay (E/T ratio, 20:1). Panel D illustrates that CAR46/28ζ T lymphocytes (▪) killed autologous and allogeneic κ+ B-CLL cells, but not allogeneic κ+ B-CLL cells. In contrast, no significant killing was observed for control transduced T lymphocytes expanded in the presence of exogenous IL-2 (□). Data represent mean ± SD of 3 different donors.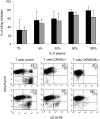
 ) or CAR46/28ζ+ (▪) were incubated with Daudi cells (E/T ratio, 20:1) in the presence of serial dilutions of plasma obtained from healthy donors. The top panel illustrates the mean ± SD of residual killing by transgenic T lymphocytes in presence of the plasma for 4 different donors. The bottom panels illustrate the effects of coculturing either control T lymphocytes or CAR46ζ+ or CAR46/28ζ+ T lymphocytes with Daudi cells at a 10:1 E/T ratio, in complete media (top panels) or in 100% human plasma (bottom panels). After 5 to 7 days of culture, cells were stained with CD3-PerCP and CD19-PE to enumerate CD19+ tumor cells. CAR46/28ζ+ T lymphocytes were able to prevent the growth of tumor cells even in the presence of human plasma. This is representative of 5 independent experiments.
) or CAR46/28ζ+ (▪) were incubated with Daudi cells (E/T ratio, 20:1) in the presence of serial dilutions of plasma obtained from healthy donors. The top panel illustrates the mean ± SD of residual killing by transgenic T lymphocytes in presence of the plasma for 4 different donors. The bottom panels illustrate the effects of coculturing either control T lymphocytes or CAR46ζ+ or CAR46/28ζ+ T lymphocytes with Daudi cells at a 10:1 E/T ratio, in complete media (top panels) or in 100% human plasma (bottom panels). After 5 to 7 days of culture, cells were stained with CD3-PerCP and CD19-PE to enumerate CD19+ tumor cells. CAR46/28ζ+ T lymphocytes were able to prevent the growth of tumor cells even in the presence of human plasma. This is representative of 5 independent experiments.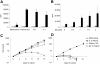
 ), and CAR46/28ζ+ T lymphocytes (▪) were incubated either with serial dilutions of plasma (A) or purified immunoglobulins (B). After 72 hours, the cells were pulsed with 1 μCi (0.037 MBq) methyl-3[H]thymidine and cultured for an additional 15 hours. Only T cells carrying the CAR46/28ζ proliferate in response to soluble immunoglobulins. Data represent mean ± SD for 3 donors. CAR46ζ+ (C) and CAR46/28ζ+ (D) T lymphocytes were also cultured for 2 weeks with the addition of plasma or soluble immunoglobulins every 3 days. Only CAR46/28ζ+ T cells expanded in presence of plasma or purified immunoglobulins. Data represent mean ± SD for 3 donors.
), and CAR46/28ζ+ T lymphocytes (▪) were incubated either with serial dilutions of plasma (A) or purified immunoglobulins (B). After 72 hours, the cells were pulsed with 1 μCi (0.037 MBq) methyl-3[H]thymidine and cultured for an additional 15 hours. Only T cells carrying the CAR46/28ζ proliferate in response to soluble immunoglobulins. Data represent mean ± SD for 3 donors. CAR46ζ+ (C) and CAR46/28ζ+ (D) T lymphocytes were also cultured for 2 weeks with the addition of plasma or soluble immunoglobulins every 3 days. Only CAR46/28ζ+ T cells expanded in presence of plasma or purified immunoglobulins. Data represent mean ± SD for 3 donors.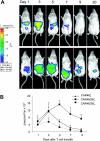

References
-
- Rohatiner AZ, Lister TA. The clinical course of follicular lymphoma. Best Pract Res Clin Haematol. 2005;18: 1-10. - PubMed
-
- Rozman C, Montserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333: 1052-1057. - PubMed
-
- Huhn D, von Schilling C, Wilhelm M, et al. Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood. 2001;98: 1326-1331. - PubMed
-
- Osterborg A, Dyer MJ, Bunjes D, et al. Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European Study Group of CAMPATH-1H Treatment in Chronic Lymphocytic Leukemia. J Clin Oncol. 1997;15: 1567-1574. - PubMed
-
- Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106: 3725-3732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

