Infection of human endothelial cells with spotted Fever group rickettsiae stimulates cyclooxygenase 2 expression and release of vasoactive prostaglandins
- PMID: 16926398
- PMCID: PMC1594856
- DOI: 10.1128/IAI.00182-06
Infection of human endothelial cells with spotted Fever group rickettsiae stimulates cyclooxygenase 2 expression and release of vasoactive prostaglandins
Abstract
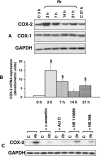
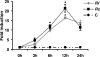
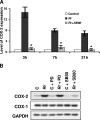
Rickettsiae, a diverse group of obligately intracellular gram-negative bacteria, include etiologic agents of the spotted fever and typhus groups of diseases. Rocky Mountain spotted fever and boutonneuse fever, due to Rickettsia rickettsii and R. conorii, respectively, are characterized by widespread infection of the vascular endothelium, microvascular injury, and vasculitis. Cultured human endothelial cells (EC) are highly susceptible to infection and respond by altering the expression of adhesion molecules, regulatory cytokines, and the antioxidant enzyme heme oxygenase (HO). In the vasculature, HO regulates the cyclooxygenase (COX) enzymes, among which the inducible isozyme COX-2 facilitates the synthesis of prostaglandins (PGs). Using in vitro and ex vivo models of infection, we demonstrate here that R. rickettsii infection of human EC causes robust induction of COX-2 mRNA and protein expression but has no apparent effect on the constitutive COX-1 isoform. Cells infected with viable rickettsiae consistently displayed significantly increased secretion of 6-keto-PGF(1alpha) and PGE(2). R. rickettsii-induced COX-2 was sensitive to inhibitors of de novo transcription and the pyridinylimidazole-based compound SB 203580, suggesting that this transcriptional host cell response involves signaling through p38 mitogen-activated protein kinase. PG production by infected cells was abrogated by NS 398 (a selective COX-2 inhibitor) and indomethacin (a pan-COX inhibitor). Immunohistochemical staining of sections of infected umbilical cords and corresponding uninfected controls revealed comparatively more intense and abundant staining for COX-2 in infected endothelia. Induction of the endothelial COX-2 system and the resultant enhanced release of vasoactive PGs may contribute to the regulation of inflammatory responses and vascular permeability changes during spotted fever rickettsioses.
Figures
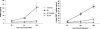

References
-
- Bach, F. H. 2005. Heme oxygenase-1: a therapeutic amplification funnel. FASEB J. 19:1216-1219. - PubMed
-
- Barnett, J., J. Chow, D. Ives, M. Chiou, R. Mackenzie, E. Osen, B. Nguyen, S. Tsing, C. Bach, J. Freire, H. Chan, E. Sigal, and C. Ramesha. 1994. Purification, characterization and selective inhibition of human prostaglandin G/H synthase 1 and 2 expressed in the baculovirus system. Biochim. Biophys. Acta 1209:130-139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

