Identification of in vivo-induced bacterial protein antigens during human infection with Salmonella enterica serovar Typhi
- PMID: 16926408
- PMCID: PMC1594849
- DOI: 10.1128/IAI.00488-06
Identification of in vivo-induced bacterial protein antigens during human infection with Salmonella enterica serovar Typhi
Abstract
We applied an immunoscreening technique, in vivo-induced antigen technology (IVIAT), to identify immunogenic bacterial proteins expressed during human infection with Salmonella enterica serovar Typhi, the cause of typhoid fever. We were able to assign a functional classification to 25 of 35 proteins identified by IVIAT. Of these 25, the majority represent proteins with known or potential roles in the pathogenesis of S. enterica. These include proteins implicated in fimbrial structure and biogenesis, antimicrobial resistance, heavy metal transport, bacterial adhesion, and extracytoplasmic substrate trafficking as well as secreted hydrolases. The 10 remaining antigens represent proteins with unknown functions. Of the 35 identified antigens, four had no immunoreactivity when probed with control sera from individuals never exposed to serovar Typhi organisms; these four included PagC, TcfB, and two antigens of unknown function encoded by STY0860 and STY3683. PagC is a virulence factor known to be upregulated in vivo in S. enterica serovar Typhimurium infection of mice. TcfB is the major structural subunit of a fimbrial operon found in serovar Typhi with no homolog in serovar Typhimurium organisms. By examining differential immunoreactivities in acute- versus convalescent-phase human serum samples, we found specific anti-PagC and anti-TcfB immunoglobulin G responses in patients with serovar Typhi bacteremia. Serovar Typhi antigens identified by IVIAT warrant further evaluation for their contributions to pathogenesis, and they may have diagnostic, therapeutic, or preventive uses.
Figures

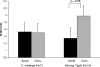
References
-
- Darwin, A. J. 2005. The phage-shock-protein response. Mol. Microbiol. 57:621-628. - PubMed
-
- Hang, L., M. John, M. Asaduzzaman, E. A. Bridges, C. Vanderspurt, T. J. Kirn, R. K. Taylor, J. D. Hillman, A. Progulske-Fox, M. Handfield, E. T. Ryan, and S. B. Calderwood. 2003. Use of in vivo-induced antigen technology (IVIAT) to identify genes uniquely expressed during human infection with Vibrio cholerae. Proc. Natl. Acad. Sci. USA 100:8508-8513. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007061/AI/NIAID NIH HHS/United States
- K12 HD 00850/HD/NICHD NIH HHS/United States
- T32 AI 07061/AI/NIAID NIH HHS/United States
- U54 AI057159/AI/NIAID NIH HHS/United States
- K01 TW007144/TW/FIC NIH HHS/United States
- K01 TW 07144/TW/FIC NIH HHS/United States
- K12 HD000850/HD/NICHD NIH HHS/United States
- AI 40725/AI/NIAID NIH HHS/United States
- R01 DE 13523/DE/NIDCR NIH HHS/United States
- R01 AI040725/AI/NIAID NIH HHS/United States
- K01 TW007409/TW/FIC NIH HHS/United States
- U01 AI 58935/AI/NIAID NIH HHS/United States
- U01 AI058935/AI/NIAID NIH HHS/United States
- R01 DE013523/DE/NIDCR NIH HHS/United States
- K01 TW 07409/TW/FIC NIH HHS/United States
- U54 AI 057159/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

