Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection
- PMID: 16926423
- PMCID: PMC1594832
- DOI: 10.1128/IAI.02024-05
Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection
Abstract
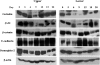
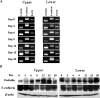
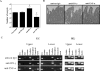
Eimeria spp. are intracellular protozoa that infect intestinal epithelia of most vertebrates, causing coccidiosis. Intestinal intraepithelial lymphocytes (IEL) that reside at the basolateral site of epithelial cells (EC) have immunoregulatory and immunoprotective roles against Eimeria spp. infection. However, it remains unknown how IEL are involved in the regulation of epithelial barrier during Eimeria sp. infection. Here, we demonstrated two distinct roles of IEL against infection with Eimeria vermiformis, a murine pathogen: production of cytokines to induce protective immunity and expression of junctional molecules to preserve epithelial barrier. The number of IEL markedly increased when oocyst production reached a peak. During infection, IEL increased production of gamma interferon (IFN-gamma) and tumor necrosis factor alpha (TNF-alpha) and decreased transforming growth factor beta (TGF-beta) production. Addition of IFN-gamma and TNF-alpha or supernatants obtained from cultured IEL from E. vermiformis-infected mice reduced transepithelial electrical resistance (TER) in a confluent CMT93 cell monolayer, a murine intestine-derived epithelial line, but antibodies against these cytokines suppressed the decline of TER. Moreover, TGF-beta attenuated the damage of epithelial monolayer and changes in TER caused by IFN-gamma and TNF-alpha. The expression of junctional molecules by EC was decreased when IEL produced a high level of IFN-gamma and TNF-alpha and a low level of TGF-beta in E. vermiformis-infected mice. Interestingly, IEL constantly expressed junctional molecules and a coculture of EC with IEL increased TER. These results suggest that IEL play important multifunctional roles not only in protection of the epithelium against E. vermiformis-induced change by cytokine production but also in direct interaction with the epithelial barrier when intra-EC junctions are down-regulated.
Figures






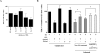
References
-
- Baumgart, D. C., W. A. Olivier, T. Reya, D. Peritt, J. L. Rombeau, and S. R. Carding. 1998. Mechanisms of intestinal epithelial cell injury and colitis in interleukin 2 (IL2)-deficient mice. Cell. Immunol. 187:52-66. - PubMed
-
- Blagburn, B. L., and K. S. Todd, Jr. 1984. Pathological changes and immunity associated with experimental Eimeria vermiformis infections in Mus musculus. J. Protozool. 31:556-561. - PubMed
-
- Chapman, H. D. 2001. Use of anticoccidial drugs in broiler chickens in the USA: analysis for the years 1995 to 1999. Poult. Sci. 80:572-580. - PubMed
-
- Chobotar, B., H. D. Danforth, and R. Entzeroth. 1993. Ultrastructural observations of host-cell invasion by sporozoites of Eimeria papillata in vivo. Parasitol. Res. 79:15-23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

