Differential availability/processing of decorin precursor in arterial and venous smooth muscle cells
- PMID: 16928198
- PMCID: PMC2100334
- DOI: 10.1111/j.1469-7580.2006.00614.x
Differential availability/processing of decorin precursor in arterial and venous smooth muscle cells
Abstract
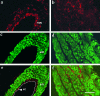
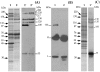
The existence of specific differentiation markers for arterial smooth muscle (SM) cells is still a matter of debate. A clone named MM1 was isolated from a library of monoclonal antibodies to adult porcine aorta, which in vivo binds to arterial but not venous SM cells, except for the pulmonary vein. MM1 immunoreactivity in Western blotting involved bands in the range of M(r) 33-226 kDa, in both arterial and venous SM tissues. However, immunoprecipitation experiments revealed that MM1 bound to a 100-kDa polypeptide that was present only in the arterial SM extract. By mass spectrometry analysis of tryptic digests from MM1-positive 130- and 120-kDa polypeptides of aorta SM extract, the antigen recognized by the antibody was identified as a decorin precursor. Using a crude decorin preparation from this tissue MM1 reacted strongly with the 33-kDa polypeptide and this pattern did not change after chondroitinase ABC treatment. In vitro, decorin immunoreactivity was found in secreted grainy material produced by confluent arterial SM cells, although lesser amounts were also seen in venous SM cells. Western blotting of extracts from these cultures showed the presence of the 33-kDa band but not of the high-molecular-weight components, except for the 100-kDa monomer. The 100/33-kDa combination was more abundant in arterial SM cells than in the venous counterpart. In the early phase of neointima formation, induced by endothelial injury of the carotid artery or vein-to-artery transposition, the decorin precursor was not expressed, but it was up-regulated in the SM cells of the media underlying the neointima in both models. Collectively, these data suggest a different processing/utilization of the 100-kDa monomer of proteoglycan decorin in arterial and venous SM cells, which is abolished after vein injury.
Figures





Similar articles
-
In vitro differences between venous and arterial-derived smooth muscle cells: potential modulatory role of decorin.Cardiovasc Res. 2005 Feb 15;65(3):702-10. doi: 10.1016/j.cardiores.2004.10.012. Cardiovasc Res. 2005. PMID: 15664397
-
Myosin isoforms and cell heterogeneity in vascular smooth muscle. I. Developing and adult bovine aorta.Dev Biol. 1990 Oct;141(2):431-46. doi: 10.1016/0012-1606(90)90398-3. Dev Biol. 1990. PMID: 2145187
-
Decorin promotes aortic smooth muscle cell calcification and colocalizes to calcified regions in human atherosclerotic lesions.Arterioscler Thromb Vasc Biol. 2004 Dec;24(12):2391-6. doi: 10.1161/01.ATV.0000147029.63303.28. Epub 2004 Oct 7. Arterioscler Thromb Vasc Biol. 2004. PMID: 15472131
-
A skeletal muscle-specific form of decorin is a target antigen for a serum IgM M-protein in a patient with a proximal myopathy.Neurology. 1997 Dec;49(6):1650-4. doi: 10.1212/wnl.49.6.1650. Neurology. 1997. PMID: 9409362
-
Myosin heavy-chain isoform composition and distribution in developing and adult human aortic smooth muscle.J Vasc Res. 1993 Sep-Oct;30(5):279-92. doi: 10.1159/000159007. J Vasc Res. 1993. PMID: 8399989
References
-
- Adams LD, Geary RL, McManus B, Schwartz SM. A comparison of aorta and vena cava medial message expression by cDNA array analysis identifies a set of 68 consistently differentially expressed genes, all in aortic media. Circ Res. 2000;87:623–631. - PubMed
-
- Ausoni S, Sartore S. Cell lineages and tissue boundaries in cardiac arterial and venous poles. Arterioscler Thromb Vasc Biol. 2001;21:312–320. - PubMed
-
- Borrione A, Zanellato AC, Scannapieco G, Pauletto P, Sartore S. Myosin heavy-chain isoforms in adult and developing rabbit vascular smooth muscle. Eur J Biochem. 1989;183:413–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

