Use of a ROSA26:GFP transgenic line for long-term Xenopus fate-mapping studies
- PMID: 16928208
- PMCID: PMC2100324
- DOI: 10.1111/j.1469-7580.2006.00608.x
Use of a ROSA26:GFP transgenic line for long-term Xenopus fate-mapping studies
Abstract
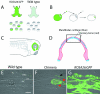
Widespread and persistent marker expression is a prerequisite for many transgenic applications, including chimeric transplantation studies. Although existing transgenic tools for the clawed frog, Xenopus laevis, offer a number of promoters that drive widespread expression during embryonic stages, obtaining transgene expression through metamorphosis and into differentiated adult tissues has been difficult to achieve with this species. Here we report the application of the murine ROSA26 promoter in Xenopus. GFP is expressed in every transgenic tissue and cell type examined at post-metamorphic stages. Furthermore, transgenic ROSA26:GFP frogs develop normally, with no apparent differences in growth or morphology relative to wild-type frogs. ROSA26 transgenes may be used as a reliable marker for embryonic fate-mapping of adult structures in Xenopus laevis. Utility of this transgenic line is illustrated by its use in a chimeric grafting study that demonstrates the derivation of the adult bony jaw from embryonic cranial neural crest.
Figures






Similar articles
-
The mouse muscle creatine kinase promoter faithfully drives reporter gene expression in transgenic Xenopus laevis.Physiol Genomics. 2004 Jun 17;18(1):79-86. doi: 10.1152/physiolgenomics.00148.2003. Epub 2004 Jun 17. Physiol Genomics. 2004. PMID: 15010518
-
A flk-1 promoter/enhancer reporter transgenic Xenopus laevis generated using the Sleeping Beauty transposon system: an in vivo model for vascular studies.Dev Dyn. 2007 Oct;236(10):2808-17. doi: 10.1002/dvdy.21321. Dev Dyn. 2007. PMID: 17879322
-
Photoreceptor development in premetamorphic and metamorphic Xenopus laevis.Anat Rec (Hoboken). 2010 Mar;293(3):383-7. doi: 10.1002/ar.21079. Anat Rec (Hoboken). 2010. PMID: 20091886
-
Transgene-driven protein expression specific to the intermediate pituitary melanotrope cells of Xenopus laevis.FEBS Lett. 2002 Apr 10;516(1-3):201-7. doi: 10.1016/s0014-5793(02)02523-1. FEBS Lett. 2002. PMID: 11959133
-
Transgenic Xenopus laevis for live imaging in cell and developmental biology.Dev Growth Differ. 2013 May;55(4):422-33. doi: 10.1111/dgd.12042. Epub 2013 Mar 11. Dev Growth Differ. 2013. PMID: 23480392 Review.
Cited by
-
Expression and loss of alleles in cultured mouse embryonic fibroblasts and stem cells carrying allelic fluorescent protein genes.BMC Mol Biol. 2006 Oct 16;7:36. doi: 10.1186/1471-2199-7-36. BMC Mol Biol. 2006. PMID: 17042952 Free PMC article.
-
pTransgenesis: a cross-species, modular transgenesis resource.Development. 2011 Dec;138(24):5451-8. doi: 10.1242/dev.066498. Development. 2011. PMID: 22110059 Free PMC article.
-
Early-Phase Luciferase Signals of Islet Grafts Predicts Successful Subcutaneous Site Transplantation in Rats.Mol Imaging Biol. 2021 Apr;23(2):173-179. doi: 10.1007/s11307-020-01560-2. Epub 2020 Nov 2. Mol Imaging Biol. 2021. PMID: 33140260 Free PMC article.
-
Red fluorescent Xenopus laevis: a new tool for grafting analysis.BMC Dev Biol. 2009 Jun 23;9:37. doi: 10.1186/1471-213X-9-37. BMC Dev Biol. 2009. PMID: 19549299 Free PMC article.
-
Cranial osteogenesis and suture morphology in Xenopus laevis: a unique model system for studying craniofacial development.PLoS One. 2009;4(1):e3914. doi: 10.1371/journal.pone.0003914. Epub 2009 Jan 19. PLoS One. 2009. PMID: 19156194 Free PMC article.
References
-
- Balaban E, Teillet M-A, Le Douarin N. Application of the quail-chick chimera system to the study of brain development and behavior. Science. 1988;241:1339–1342. - PubMed
-
- Billington N, Knight AW. Seeing the wood through the trees: a review of techniques for distinguishing green fluorescent protein from endogenous autofluorescence. Anal Biochem. 2001;291:175–197. - PubMed
-
- Chai Y, Jiang X, Ito Y, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. - PubMed
-
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

