External tufted cells in the main olfactory bulb form two distinct subpopulations
- PMID: 16930438
- PMCID: PMC1557706
- DOI: 10.1111/j.1460-9568.2006.04988.x
External tufted cells in the main olfactory bulb form two distinct subpopulations
Abstract
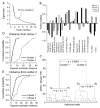
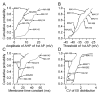
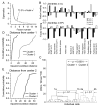
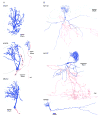
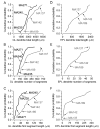
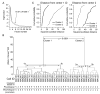
The glomeruli of the main olfactory bulb are the first processing station of the olfactory pathway, where complex interactions occur between sensory axons, mitral cells and a variety of juxtaglomerular neurons, including external tufted cells (ETCs). Despite a number of studies characterizing ETCs, little is known about how their morphological and functional properties correspond to each other. Here we determined the active and passive electrical properties of ETCs using in vitro whole-cell recordings, and correlated them with their dendritic arborization patterns. Principal component followed by cluster analysis revealed two distinct subpopulations of ETCs based on their electrophysiological properties. Eight out of 12 measured physiological parameters exhibited significant difference between the two subpopulations, including the membrane time constant, amplitude of spike afterhyperpolarization, variance in the interspike interval distribution and subthreshold resonance. Cluster analysis of the morphological properties of the cells also revealed two subpopulations, the most prominent dissimilarity between the groups being the presence or absence of secondary, basal dendrites. Finally, clustering the cells taking all measured properties into account also indicated the presence of two subpopulations that mapped in an almost perfect one-to-one fashion to both the physiologically and the morphologically derived groups. Our results demonstrate that a number of functional and structural properties of ETCs are highly predictive of one another. However, cells within each subpopulation exhibit pronounced variability, suggesting a large degree of specialization evolved to fulfil specific functional requirements in olfactory information processing.
Figures
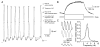

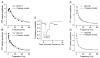
References
-
- Adrian ED. The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol. 1950;2:377–388. - PubMed
-
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. - PubMed
-
- Cajal, S.R. & y. (1911) Histologie Du Systeme Nerveux de l’Homme et Des Vertebres Maloine, Paris.
-
- Freeman, W.J. (1975) Mass Action in the Nervous System Academic Press, New York.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

