The fission yeast DNA structure checkpoint protein Rad26ATRIP/LCD1/UVSD accumulates in the cytoplasm following microtubule destabilization
- PMID: 16930478
- PMCID: PMC1592483
- DOI: 10.1186/1471-2121-7-32
The fission yeast DNA structure checkpoint protein Rad26ATRIP/LCD1/UVSD accumulates in the cytoplasm following microtubule destabilization
Abstract
Background: DNA structure checkpoints are conserved eukaryotic signal transduction pathways that help preserve genomic integrity. Upon detecting checkpoint signals such as stalled replication forks or double-stranded DNA breaks, these pathways coordinate appropriate stress responses. Members of the PI-3 kinase related kinase (PIKK) family are essential elements of DNA structure checkpoints. In fission yeast, the Rad3 PIKK and its regulatory subunit Rad26 coordinate the detection of checkpoint signals with pathway outputs.
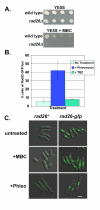
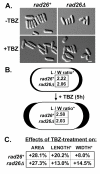
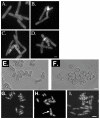
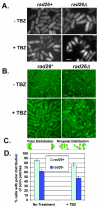
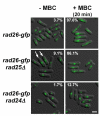
Results: We found that untreated rad26Delta cells were defective for two microtubule-dependent processes: chromosome segregation and morphogenesis. Interestingly, cytoplasmic accumulation of Rad26-GFP occurred following treatment with microtubule destabilizing drugs, but not during treatment with the genotoxic agent Phleomycin. Cytoplasmic accumulation of Rad26-GFP depended on Rad24, a 14-3-3 protein also required for DNA structure checkpoints and morphogenesis. Results of over expression and epistasis experiments confirm that Rad26 and Rad24 define a response to microtubule destabilizing conditions.
Conclusion: Two DNA structure checkpoint proteins with roles in morphogenesis define a response to microtubule destabilizing conditions.
Figures



Similar articles
-
Fission yeast Rad26ATRIP delays spindle-pole-body separation following interphase microtubule damage.J Cell Sci. 2010 May 1;123(Pt 9):1537-45. doi: 10.1242/jcs.049478. Epub 2010 Apr 7. J Cell Sci. 2010. PMID: 20375067
-
Fission yeast Rad26 responds to DNA damage independently of Rad3.BMC Genet. 2003 Apr 3;4:6. doi: 10.1186/1471-2156-4-6. BMC Genet. 2003. PMID: 12697061 Free PMC article.
-
Long G2 accumulates recombination intermediates and disturbs chromosome segregation at dysfunction telomere in Schizosaccharomyces pombe.Biochem Biophys Res Commun. 2015 Aug 14;464(1):140-6. doi: 10.1016/j.bbrc.2015.06.098. Epub 2015 Jun 18. Biochem Biophys Res Commun. 2015. PMID: 26093291
-
Rad3 and Sty1 function in Schizosaccharomyces pombe: an integrated response to DNA damage and environmental stress?Mol Microbiol. 2008 Apr;68(2):246-54. doi: 10.1111/j.1365-2958.2008.06147.x. Mol Microbiol. 2008. PMID: 18366437 Review.
-
Linking the organization of DNA replication with genome maintenance.Curr Genet. 2019 Jun;65(3):677-683. doi: 10.1007/s00294-018-0923-8. Epub 2019 Jan 2. Curr Genet. 2019. PMID: 30600398 Review.
Cited by
-
Effect of Surface Coating of Gold Nanoparticles on Cytotoxicity and Cell Cycle Progression.Nanomaterials (Basel). 2018 Dec 17;8(12):1063. doi: 10.3390/nano8121063. Nanomaterials (Basel). 2018. PMID: 30562921 Free PMC article.
-
A genomic Multiprocess survey of machineries that control and link cell shape, microtubule organization, and cell-cycle progression.Dev Cell. 2014 Oct 27;31(2):227-239. doi: 10.1016/j.devcel.2014.09.005. Dev Cell. 2014. PMID: 25373780 Free PMC article.
-
Pap1 + confers microtubule damage resistance to mut2a, an extragenic suppressor of the rad26:4A allele in S. pombe.Mol Biol Res Commun. 2018 Sep;7(3):97-106. doi: 10.22099/mbrc.2018.29705.1324. Mol Biol Res Commun. 2018. PMID: 30426027 Free PMC article.
-
Fission yeast Ase1PRC1 is required for the G2-microtubule damage response.Mol Biol Res Commun. 2021 Dec;10(4):179-188. doi: 10.22099/mbrc.2021.41001.1650. Mol Biol Res Commun. 2021. PMID: 35097140 Free PMC article.
References
-
- Carr AM. DNA structure dependent checkpoints as regulators of DNA repair. DNA Repair (Amst) 2002;1:983–994. - PubMed
-
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. - PubMed
-
- Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. - PubMed
-
- Edwards RJ, Bentley NJ, Carr AM. A Rad3-Rad26 complex responds to DNA damage independently of other checkpoint proteins. Nat Cell Biol. 1999;1:393–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

