Peripheral amplification of sweating--a role for calcitonin gene-related peptide
- PMID: 16931551
- PMCID: PMC1890409
- DOI: 10.1113/jphysiol.2006.116111
Peripheral amplification of sweating--a role for calcitonin gene-related peptide
Abstract
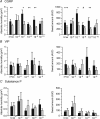
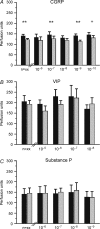
Neuropeptides are the mediators of neurogenic inflammation. Some pain disorders, e.g. complex regional pain syndromes, are characterized by increased neurogenic inflammation and by exaggerated sudomotor function. The aim of this study was to explore whether neuropeptides have a peripheral effect on human sweating. We investigated the effects of different concentrations of calcitonin gene-related peptide (CGRP), vasoactive intestinal peptide (VIP) and substance P (SP) on acetylcholine-induced axon reflex sweating in healthy subjects (total n = 18). All substances were applied via dermal microdialysis. The experiments were done in a parallel setting: ACh alone and ACh combined with CGRP, VIP or SP in various concentrations were applied. Acetylcholine (10(-2) m) always elicited a sweating response, neuropeptides alone did not. However, CGRP significantly enhanced ACh-induced sweating (P < 0.01). Post hoc tests revealed that CGRP in physiological concentrations of 10(-7)-10(-9) m was most effective. VIP at any concentration had no significant effect on axon reflex sweating. The duration of the sweating response (P < 0.01), but not the amount of sweat, was reduced by SP. ACh-induced skin blood flow was significantly increased by CGRP (P < 0.01), but unaltered by VIP and SP. The results indicate that CGRP amplifies axon reflex sweating in human skin.
Figures

Similar articles
-
Inhibition of neuropeptide degradation suppresses sweating but increases the area of the axon reflex flare.Exp Dermatol. 2013 Apr;22(4):299-301. doi: 10.1111/exd.12122. Exp Dermatol. 2013. PMID: 23528219
-
Modulation of the acetylcholine- and substance P-induced pulmonary edema by calcitonin gene-related peptide in the rabbit.J Pharmacol Exp Ther. 1994 Jul;270(1):30-6. J Pharmacol Exp Ther. 1994. PMID: 7518513
-
Interaction of calcitonin gene related peptide (CGRP) and substance P (SP) in human skin.Neuropeptides. 2016 Oct;59:57-62. doi: 10.1016/j.npep.2016.06.001. Epub 2016 Jun 18. Neuropeptides. 2016. PMID: 27344069
-
Neuropeptides and asthma.Am Rev Respir Dis. 1991 Mar;143(3 Pt 2):S28-32. doi: 10.1164/ajrccm/143.3_Pt_2.S28. Am Rev Respir Dis. 1991. PMID: 1706152 Review.
-
Neurogenic vasodilatation and plasma leakage in the skin.Gen Pharmacol. 1998 Jan;30(1):5-11. doi: 10.1016/s0306-3623(97)00078-5. Gen Pharmacol. 1998. PMID: 9457475 Review.
Cited by
-
Primary hyperhidrosis: From a genetics point of view.J Family Med Prim Care. 2023 Dec;12(12):3028-3032. doi: 10.4103/jfmpc.jfmpc_1568_22. Epub 2023 Dec 21. J Family Med Prim Care. 2023. PMID: 38361865 Free PMC article. Review.
-
From a Symptom-Based to a Mechanism-Based Pharmacotherapeutic Treatment in Complex Regional Pain Syndrome.Drugs. 2022 Apr;82(5):511-531. doi: 10.1007/s40265-022-01685-4. Epub 2022 Mar 5. Drugs. 2022. PMID: 35247200 Free PMC article. Review.
-
Neuropeptide PACAP promotes sweat secretion.Br J Dermatol. 2017 Feb;176(2):295-296. doi: 10.1111/bjd.15162. Br J Dermatol. 2017. PMID: 28244081 Free PMC article. No abstract available.
-
Complex regional pain syndrome: a focus on the autonomic nervous system.Clin Auton Res. 2019 Aug;29(4):457-467. doi: 10.1007/s10286-019-00612-0. Epub 2019 May 18. Clin Auton Res. 2019. PMID: 31104164 Review.
-
Efficacy and safety of ketamine in patients with complex regional pain syndrome: a systematic review.CNS Drugs. 2012 Mar 1;26(3):215-28. doi: 10.2165/11595200-000000000-00000. CNS Drugs. 2012. PMID: 22136149
References
-
- Bennett AD, Chastain KM, Hulsebosch CE. Alleviation of mechanical and thermal allodynia by CGRP8–37 in a rodent model of chronic central pain. Pain. 2000;86:163–175. - PubMed
-
- Berg TJ, Levy DM, Reid G, Abraham RR. The effects of vasoactive intestinal polypeptide and substance P on methacholine-induced sweating and vascular flare in diabetic neuropathy. Clin Auton Res. 1995;5:159–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

