BCR ubiquitination controls BCR-mediated antigen processing and presentation
- PMID: 16931624
- PMCID: PMC1895444
- DOI: 10.1182/blood-2006-05-025338
BCR ubiquitination controls BCR-mediated antigen processing and presentation
Abstract
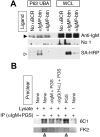
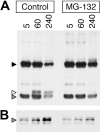
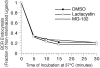
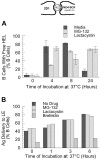
BCR-mediated antigen processing occurs at immunologically relevant antigen concentrations and hinges on the trafficking of antigen-BCR (Ag-BCR) complexes to class II-containing multivesicular bodies (MVBs) termed MIICs. However, the molecular mechanism underlying the trafficking of Ag-BCR complexes to and within MIICs is not well understood. In contrast, the trafficking of the epidermal growth factor receptor (EGFR) to and within MVBs occurs via a well-characterized ubiquitin-dependent mechanism, which is blocked by acute inhibition of proteasome activity. Using a highly characterized antigen-specific model system, it was determined that the immunoglobulin heavy chain subunit of the IgM BCR of normal (ie, nontransformed) B cells is ubiquitinated. Moreover, acute inhibition of proteasome activity delays the formation of ubiquitinated ligand-BCR complexes, alters the intracellular trafficking of internalized Ag-BCR complexes, and selectively blocks the BCR-mediated processing and presentation of cognate antigen, without inhibiting the endocytosis, processing, and presentation of non-cognate antigen internalized by fluidphase endocytosis. These results demonstrate that the trafficking of Ag-BCR complexes to and within MVB-like antigen processing compartments occurs via a molecular mechanism with similarities to that used by the EGFR, and establishes the EGFR as a paradigm for the further analysis of Ag-BCR trafficking to and within MIICs.
Figures


References
-
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23: 975-1028. - PubMed
-
- Kleijmeer MJ, Raposo G, Geuze HJ. Characterization of MHC class II compartments by immunoelectron microscopy. Methods. 1996;10: 191-207. - PubMed
-
- Watts C. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat Immunol. 2004; 5: 685-692. - PubMed
-
- Watts C, Reid PA, West MA, Davidson HW. The antigen processing pathway in B lymphocytes. Semin Immunol. 1990;2: 247-253. - PubMed
-
- Nashar TO, Drake JR. The pathway of antigen uptake and processing dictates MHC class II-mediated B cell survival and activation. J Immunol. 2005;174: 1306-1316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

