Symbiotic bacteria direct expression of an intestinal bactericidal lectin
- PMID: 16931762
- PMCID: PMC2716667
- DOI: 10.1126/science.1127119
Symbiotic bacteria direct expression of an intestinal bactericidal lectin
Abstract
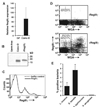
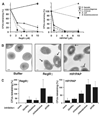
The mammalian intestine harbors complex societies of beneficial bacteria that are maintained in the lumen with minimal penetration of mucosal surfaces. Microbial colonization of germ-free mice triggers epithelial expression of RegIIIgamma, a secreted C-type lectin. RegIIIgamma binds intestinal bacteria but lacks the complement recruitment domains present in other microbe-binding mammalian C-type lectins. We show that RegIIIgamma and its human counterpart, HIP/PAP, are directly antimicrobial proteins that bind their bacterial targets via interactions with peptidoglycan carbohydrate. We propose that these proteins represent an evolutionarily primitive form of lectin-mediated innate immunity, and that they reveal intestinal strategies for maintaining symbiotic host-microbial relationships.
Figures


Comment in
-
Immunology. Unraveling gut inflammation.Science. 2006 Aug 25;313(5790):1052-4. doi: 10.1126/science.1131997. Science. 2006. PMID: 16931742 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

