Contractility and Ca2+ signaling of smooth muscle cells in different generations of mouse airways
- PMID: 16931808
- PMCID: PMC1899303
- DOI: 10.1165/rcmb.2006-0036OC
Contractility and Ca2+ signaling of smooth muscle cells in different generations of mouse airways
Abstract
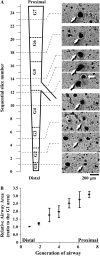
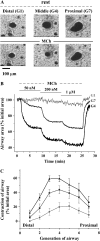
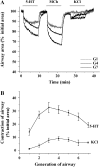
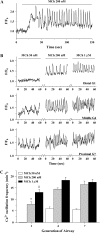
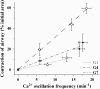
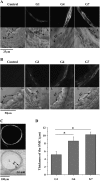
The control and mechanisms of airway smooth muscle cell (SMC) contraction were investigated with a sequential series of lung slices from different generations of the same airway from the cardiac lobe of the mouse lung. Airway contraction was measured by monitoring the changes in airway lumen area with phase-contrast microscopy. Changes in intracellular calcium concentration of the SMCs were studied with a custom-built confocal or two-photon microscope. The distribution of the airway SMCs and the muscarinic M(3) or 5-HT(2A) receptors was determined with immunofluorescence. Methacholine and 5-HT induced a concentration-dependent airway contraction and Ca(2+) oscillations within the SMCs of each airway generation. The airway contraction in response to the same agonist concentration was greater in the middle generation compared with the distal or proximal generations of the same airway. Similarly, the Ca(2+) oscillations varied in different generations of the same airway, with a slower frequency in the SMCs of the distal zone as compared with the middle or proximal zones of airways. By contrast, high KCl induced minimal contraction and very slow Ca(2+) oscillations throughout the whole intrapulmonary airway. The slower agonist-induced Ca(2+) oscillations in the distal zone correlated with a reduced expression of agonist receptors. The layer of SMCs increased in thickness in the middle and proximal zones. These results indicate that the contractility of airway SMCs varies at different positions along the same airway and that this response partially results from different Ca(2+) signaling and the total amount of the SMCs.
Figures

References
-
- Global Initiative for Asthma. Global Strategy for asthma management and prevention. National Institute of Health 2002, NIH publication no. 02-3659.
-
- Hamid Q, Song Y, Kotsimbos TC, Minshall E, Bai TR, Hegele RG, Hogg JC. Inflammation of small airways in asthma. J Allergy Clin Immunol 1997;100:44–51. - PubMed
-
- Martin RJ. Therapeutic significance of distal airway inflammation in asthma. J Allergy Clin Immunol 2002;109:S447–S460. - PubMed
-
- Roche WR. Inflammatory and structural changes in the small airways in bronchial asthma. Am J Respir Crit Care Med 1998;157:S191–S194. - PubMed
-
- Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol 1992;72:1016–1023. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

