Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility
- PMID: 16932748
- PMCID: PMC1560351
- DOI: 10.1038/sj.emboj.7601272
Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility
Abstract
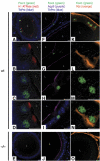
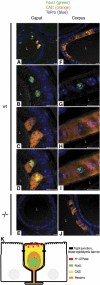
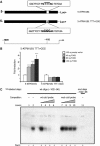
An essential aspect of male reproductive capacity is the immediate availability of fertilization-ready spermatozoa. To ensure this, most mammals rely on post-testicular sperm maturation. In epididymis, germ cells are matured and stored in a quiescent state that readily can be altered to produce active spermatozoa. This depends on active proton secretion into the epididymal lumen. We have identified Foxi1 as an important regulator of gene expression in narrow and clear cells-the major proton secretory cells of epididymal epithelia. Foxi1 appears to be required for the expression of the B1-subunit of the vacuolar H+ -ATPase proton pump and for carbonic anhydrase II as well as the chloride/bicarbonate transporter pendrin. Using transfection experiments, we have identified a Foxi1 binding cis-element in the ATP6V1B1 (encoding the B1-subunit) promoter that is critical for reporter gene activation. When this site is mutated to eliminate Foxi1 binding, activation is also abolished. As a consequence of defect Foxi1-dependent epididymal sperm maturation, we demonstrate that spermatozoa from Foxi1 null males fail to reach the female genital tract in sufficient number to allow fertilization.
Figures




References
-
- Acott TS, Carr DW (1984) Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor. Biol Reprod 30: 926–935 - PubMed
-
- Al-Awqati Q (1996) Plasticity in epithelial polarity of renal intercalated cells: targeting of the H(+)-ATPase and band 3. Am J Physiol 270: C1571–C1580 - PubMed
-
- Bedford JM (1975) Maturation, transport, and fate of spermatozoa in the epididymis. In Handbook of Physiology Section 7: Endocrinolology, Vol. V. Male Reproductive System, Greep RO, Astwood EB (eds). pp 303–317. Washington DC: American Physiology Society
-
- Breton S, Smith PJ, Lui B, Brown D (1996) Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med 2: 470–472 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

