Frequency of adverse events after vaccination with different vaccinia strains
- PMID: 16933957
- PMCID: PMC1551910
- DOI: 10.1371/journal.pmed.0030272
Frequency of adverse events after vaccination with different vaccinia strains
Erratum in
- PLoS Med. 2006 Oct;3(10):e429
Abstract
Background: Large quantities of smallpox vaccine have been stockpiled to protect entire nations against a possible reintroduction of smallpox. Planning for an appropriate use of these stockpiled vaccines in response to a smallpox outbreak requires a rational assessment of the risks of vaccination-related adverse events, compared to the risk of contracting an infection. Although considerable effort has been made to understand the dynamics of smallpox transmission in modern societies, little attention has been paid to estimating the frequency of adverse events due to smallpox vaccination. Studies exploring the consequences of smallpox vaccination strategies have commonly used a frequency of approximately one death per million vaccinations, which is based on a study of vaccination with the New York City Board of Health (NYCBH) strain of vaccinia virus. However, a multitude of historical studies of smallpox vaccination with other vaccinia strains suggest that there are strain-related differences in the frequency of adverse events after vaccination. Because many countries have stockpiled vaccine based on the Lister strain of vaccinia virus, a quantitative evaluation of the adverse effects of such vaccines is essential for emergency response planning. We conducted a systematic review and statistical analysis of historical data concerning vaccination against smallpox with different strains of vaccinia virus.
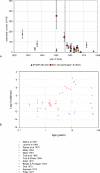
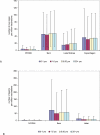
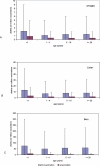
Methods and findings: We analyzed historical vaccination data extracted from the literature. We extracted data on the frequency of postvaccinal encephalitis and death with respect to vaccinia strain and age of vaccinees. Using a hierarchical Bayesian approach for meta-analysis, we estimated the expected frequencies of postvaccinal encephalitis and death with respect to age at vaccination for smallpox vaccines based on the NYCBH and Lister vaccinia strains. We found large heterogeneity between findings from different studies and a time-period effect that showed decreasing incidences of adverse events over several decades. To estimate death rates, we then restricted our analysis to more-recent studies. We estimated that vaccination with the NYCBH strain leads to an average of 1.4 deaths per million vaccinations (95% credible interval, 0-6) and that vaccination with Lister vaccine leads to an average of 8.4 deaths per million vaccinations (95% credible interval, 0-31). We combined age-dependent estimates of the frequency of death after vaccination and revaccination with demographic data to obtain estimates of the expected number of deaths in present societies due to vaccination with the NYCBH and Lister vaccinia strains.
Conclusions: Previous analyses of smallpox vaccination policies, which rely on the commonly assumed value of one death per million vaccinations, may give serious underestimates of the number of deaths resulting from vaccination. Moreover, because there are large, strain-dependent differences in the frequency of adverse events due to smallpox vaccination, it is difficult to extrapolate from predictions for the NYCBH-derived vaccines (stockpiled in countries such as the US) to predictions for the Lister-derived vaccines (stockpiled in countries such as Germany). In planning for an effective response to a possible smallpox outbreak, public-health decision makers should reconsider their strategies of when to opt for ring vaccination and when to opt for mass vaccination.
Conflict of interest statement
Figures

References
-
- Abrahams BC, Kaufman DM. Anticipating smallpox and monkeypox outbreaks: Complications of the smallpox vaccine. Neurologist. 2004;10:265–274. - PubMed
-
- Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: A review, part II. Adverse events. Clin Infect Dis. 2003;37:251–271. - PubMed
-
- Morita M, Aoyama Y, Arita M, Amona H, Yoshizawa H, et al. Comparative studies of several vaccinia virus strains by intrathalamic inoculation into cynomolgus monkeys. Arch Virol. 1977;53:197. - PubMed
-
- Marennikova SS. Evaluation of vaccine strains by their behaviour in vaccinated animals and possible implications of the revealed features for smallpox vaccination practice. In: Regamey RH, Cohen H, editors. Symposia Series in Immunobiological Standardization. Volume 19. Basel: Karger; 1974. pp. 253–260.
-
- Poland GA, Grabenstein JD, Neff JM. The US smallpox vaccination program: A review of a large modern era smallpox vaccination implementation program. Vaccine. 2005;23:2078. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

