Disruption-induced mucus secretion: repair and protection
- PMID: 16933971
- PMCID: PMC1544361
- DOI: 10.1371/journal.pbio.0040276
Disruption-induced mucus secretion: repair and protection
Abstract
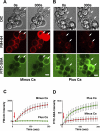
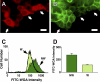
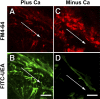
When a cell suffers a plasma membrane disruption, extracellular Ca(2+) rapidly diffuses into its cytosol, triggering there local homotypic and exocytotic membrane fusion events. One role of this emergency exocytotic response is to promote cell survival: the internal membrane thus added to the plasma membrane acts as a reparative "patch." Another, unexplored consequence of disruption-induced exocytosis is secretion. Many of the cells lining the gastrointestinal tract secrete mucus via a compound exocytotic mechanism, and these and other epithelial cell types lining the digestive tract are normally subject to plasma membrane disruption injury in vivo. Here we show that plasma membrane disruption triggers a potent mucus secretory response from stomach mucous cells wounded in vitro by shear stress or by laser irradiation. This disruption-induced secretory response is Ca(2+) dependent, and coupled to cell resealing: disruption in the absence of Ca(2+) does not trigger mucus release, but results instead in cell death due to failure to reseal. Ca(2+)-dependent, disruption-induced mucus secretion and resealing were also demonstrable in segments of intact rat large intestine. We propose that, in addition to promoting cell survival of membrane disruptions, disruption-induced exocytosis serves also the important protective function of liberating lubricating mucus at sites of mechanical wear and tear. This mode of mechanotransduction can, we propose, explain how lubrication in the gastrointestinal tract is rapidly and precisely adjusted to widely fluctuating, diet-dependent levels of mechanical stress.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures



Comment in
-
The path to digestion is paved with repair.PLoS Biol. 2006 Sep;4(9):e307. doi: 10.1371/journal.pbio.0040307. Epub 2006 Aug 22. PLoS Biol. 2006. PMID: 20076638 Free PMC article. No abstract available.
References
-
- McNeil PL, Steinhardt RA. Plasma membrane disruption: Repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. - PubMed
-
- McNeil PL, S, Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology. 1989;96:1238–1248. - PubMed
-
- Forstner G. Signal transduction, packaging and secretion of mucins. Annu Rev Physiol. 1995;57:585–605. - PubMed
-
- Bickel M, Kauffman GL., Jr Gastric gel mucus thickness: Effect of distention, 16,16-dimethyl prostaglandin e2, and carbenoxolone. Gastroenterology. 1981;80:770–775. - PubMed
-
- Enss ML, Schmidt-Wittig U, Honer K, Kownatzki R, Gartner K. Mechanical challenge causes alterations of rat colonic mucosa and released mucins. Alterations of mucosa and mucins. J Exp Anim Sci. 1994;36:128–140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

