Toxoplasma MIC2 is a major determinant of invasion and virulence
- PMID: 16933991
- PMCID: PMC1550269
- DOI: 10.1371/journal.ppat.0020084
Toxoplasma MIC2 is a major determinant of invasion and virulence
Abstract
Like its apicomplexan kin, the obligate intracellular protozoan Toxoplasma gondii actively invades mammalian cells and uses a unique form of gliding motility. The recent identification of several transmembrane adhesive complexes, potentially capable of gripping external receptors and the sub-membrane actinomyosin motor, suggests that the parasite has multiple options for host-cell recognition and invasion. To test whether the transmembrane adhesin MIC2, together with its partner protein M2AP, participates in a major invasion pathway, we utilized a conditional expression system to introduce an anhydrotetracycline-responsive mic2 construct, allowing us to then knockout the endogenous mic2 gene. Conditional suppression of MIC2 provided the first opportunity to directly determine the role of this protein in infection. Reduced MIC2 expression resulted in mistrafficking of M2AP, markedly defective host-cell attachment and invasion, the loss of helical gliding motility, and the inability to support lethal infection in a murine model of acute toxoplasmosis. Survival of mice infected with MIC2-deficient parasites correlated with lower parasite burden in infected tissues, an attenuated inflammatory immune response, and induction of long-term protective immunity. Our findings demonstrate that the MIC2 protein complex is a major virulence determinant for Toxoplasma infection and that MIC2-deficient parasites constitute an effective live-attenuated vaccine for experimental toxoplasmosis.
Conflict of interest statement
Figures



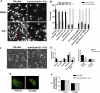

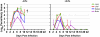

References
-
- Alaeddine F, Keller N, Leepin A, Hemphill A. Reduced infection and protection from clinical signs of cerebral neosporosis in C57BL/6 mice vaccinated with recombinant microneme antigen NcMIC1. J Parasitol. 2005;91:657–665. - PubMed
-
- Tomley FM, Billington KJ, Bumstead JM, Clark JD, Monaghan P. EtMIC4: A microneme protein from Eimeria tenella that contains tandem arrays of epidermal growth factor-like repeats and thrombospondin type-I repeats. Int J Parasitol. 2001;31:1303–1310. - PubMed
-
- Dubremetz JF, Garcia-Reguet N, Conseil V, Fourmaux MN. Apical organelles and host-cell invasion by Apicomplexa . Int J Parasitol. 1998;28:1007–1013. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

