Isotope-edited IR spectroscopy for the study of membrane proteins
- PMID: 16935550
- PMCID: PMC7185810
- DOI: 10.1016/j.cbpa.2006.08.013
Isotope-edited IR spectroscopy for the study of membrane proteins
Abstract
Fourier transform infrared (FTIR) spectroscopy has long been a powerful tool for structural analysis of membrane proteins. However, because of difficulties in resolving contributions from individual residues, most of the derived measurements tend to yield average properties for the system under study. Isotope editing, through its ability to resolve individual vibrations, establishes FTIR as a method that is capable of yielding accurate structural data on individual sites in a protein.
Figures

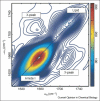
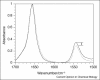
 16O label (dashed line) or a single 13C
16O label (dashed line) or a single 13C 18O label (black line). The location of the unlabeled and labeled peaks are shown.
18O label (black line). The location of the unlabeled and labeled peaks are shown.

References
-
- Krimm S., Bandekar J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv Protein Chem. 1986;38:181–364. - PubMed
-
- Braiman M.S., Rothschild K.J. Fourier transform infrared techniques for probing membrane protein structure. Annu Rev Biophys Biophys Chem. 1988;17:541–570. - PubMed
-
- Surewicz W.K., Mantsch H.H., Chapman D. Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry. 1993;32:389–394. - PubMed
-
- Tatulian S.A. Attenuated total reflection Fourier transform infrared spectroscopy: a method of choice for studying membrane proteins and lipids. Biochemistry. 2003;42:11898–11907. - PubMed
-
- Wallace B.A., Teeters C.L. Differential absorption flattening optical effects are significant in the circular dichroism spectra of large membrane fragments. Biochemistry. 1987;26:65–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

