Snf2 family ATPases and DExx box helicases: differences and unifying concepts from high-resolution crystal structures
- PMID: 16935875
- PMCID: PMC1616948
- DOI: 10.1093/nar/gkl540
Snf2 family ATPases and DExx box helicases: differences and unifying concepts from high-resolution crystal structures
Abstract
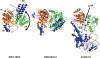
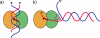
Proteins with sequence similarity to the yeast Snf2 protein form a large family of ATPases that act to alter the structure of a diverse range of DNA-protein structures including chromatin. Snf2 family enzymes are related in sequence to DExx box helicases, yet they do not possess helicase activity. Recent biochemical and structural studies suggest that the mechanism by which these enzymes act involves ATP-dependent translocation on DNA. Crystal structures suggest that these enzymes travel along the minor groove, a process that can generate the torque or energy in remodelling processes. We review the recent structural and biochemical findings which suggest a common mechanistic basis underlies the action of many of both Snf2 family and DExx box helicases.
Figures
Similar articles
-
Structural insights into regulation and action of SWI2/SNF2 ATPases.Curr Opin Struct Biol. 2011 Dec;21(6):719-27. doi: 10.1016/j.sbi.2011.09.003. Epub 2011 Oct 11. Curr Opin Struct Biol. 2011. PMID: 21996440 Free PMC article. Review.
-
X-ray structures of the Sulfolobus solfataricus SWI2/SNF2 ATPase core and its complex with DNA.Cell. 2005 May 6;121(3):363-73. doi: 10.1016/j.cell.2005.03.026. Cell. 2005. PMID: 15882619
-
Structure-function analysis of SWI2/SNF2 enzymes.Methods Enzymol. 2006;409:375-88. doi: 10.1016/S0076-6879(05)09022-1. Methods Enzymol. 2006. PMID: 16793413
-
Mechanisms of nucleic acid translocases: lessons from structural biology and single-molecule biophysics.Curr Opin Struct Biol. 2007 Feb;17(1):87-95. doi: 10.1016/j.sbi.2006.11.003. Epub 2006 Dec 6. Curr Opin Struct Biol. 2007. PMID: 17157498 Review.
-
Crystal structure of a DExx box DNA helicase.Nature. 1996 Nov 28;384(6607):379-83. doi: 10.1038/384379a0. Nature. 1996. PMID: 8934527
Cited by
-
Structural insights into regulation and action of SWI2/SNF2 ATPases.Curr Opin Struct Biol. 2011 Dec;21(6):719-27. doi: 10.1016/j.sbi.2011.09.003. Epub 2011 Oct 11. Curr Opin Struct Biol. 2011. PMID: 21996440 Free PMC article. Review.
-
The BRG1 ATPase of human SWI/SNF chromatin remodeling enzymes as a driver of cancer.Epigenomics. 2017 Jun;9(6):919-931. doi: 10.2217/epi-2017-0034. Epub 2017 May 19. Epigenomics. 2017. PMID: 28521512 Free PMC article. Review.
-
Histone protein surface accessibility dictates direction of RSC-dependent nucleosome mobilization.Nucleic Acids Res. 2022 Oct 14;50(18):10376-10384. doi: 10.1093/nar/gkac790. Nucleic Acids Res. 2022. PMID: 36161493 Free PMC article.
-
Intrinsically Disordered Regions Define Unique Protein Interaction Networks in CHD Family Remodelers.FASEB J. 2025 May 31;39(10):e70632. doi: 10.1096/fj.202402808RR. FASEB J. 2025. PMID: 40372282 Free PMC article.
-
A genome-wide survey and systematic RNAi-based characterization of helicase-like genes in Caenorhabditis elegans.DNA Res. 2007 Aug 31;14(4):183-99. doi: 10.1093/dnares/dsm016. Epub 2007 Oct 6. DNA Res. 2007. PMID: 17921522 Free PMC article.
References
-
- Becker P.B., Horz W. ATP-dependent nucleosome remodeling. Annu. Rev. Biochem. 2002;71:247–273. - PubMed
-
- Mannhaupt G., Stucka R., Ehnle S., Vetter I., Feldmann H. Molecular analysis of yeast chromosome II between CMD1 and LYS2: the excision repair gene RAD16 located in this region belongs to a novel group of double-finger proteins. Yeast. 1992;8:397–408. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

