Remodeling of ribonucleoprotein complexes with DExH/D RNA helicases
- PMID: 16935886
- PMCID: PMC1616955
- DOI: 10.1093/nar/gkl410
Remodeling of ribonucleoprotein complexes with DExH/D RNA helicases
Abstract
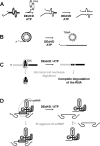
The DExH/D protein family is the largest group of enzymes in eukaryotic RNA metabolism. DExH/D proteins are mainly known for their ability to unwind RNA duplexes in an ATP-dependent fashion. However, it has become clear in recent years that these DExH/D RNA helicases are also involved in the ATP-dependent remodeling of RNA-protein complexes. Here we review recent studies that highlight physiological roles of DExH/D proteins in the displacement of proteins from RNA. We further discuss work with simple RNA-protein complexes in vitro, which illuminates mechanisms by which DExH/D proteins remove proteins from RNA. Although we are only beginning to understand how DExH/D proteins remodel RNA-protein complexes, these studies have shown that an 'RNA helicase' does not per se require cofactors to displace proteins from RNA, that protein displacement does not necessarily involve RNA duplex unwinding, and that not all DExH/D proteins are able to disassemble the same range of ribonucleoproteins.
Figures

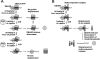
References
-
- Reed R. Coupling transcription, splicing and mRNA export. Curr. Opin. Cell. Biol. 2003;15:326–331. - PubMed
-
- Beggs J.D., Tollervey D. Crosstalk between RNA metabolic pathways: an RNOMICS approach. Nature Rev. Mol. Cell Biol. 2005;6:423–429. - PubMed
-
- Linder P. The life of RNA with proteins. Science. 2004;304:694–695. - PubMed
-
- Nilsen T.W. The spliceosome: the most complex macromolecular machine in the cell? Bioessays. 2003;25:1147–1149. - PubMed

