Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion
- PMID: 16936041
- PMCID: PMC1636320
- DOI: 10.1128/JB.00673-06
Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion
Abstract
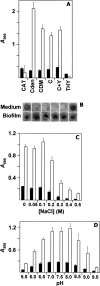
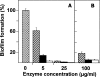
Streptococcus pneumoniae colonizes the human upper respiratory tract, and this asymptomatic colonization is known to precede pneumococcal disease. In this report, chemically defined and semisynthetic media were used to identify the initial steps of biofilm formation by pneumococcus during growth on abiotic surfaces such as polystyrene or glass. Unencapsulated pneumococci adhered to abiotic surfaces and formed a three-dimensional structure about 25 microm deep, as observed by confocal laser scanning microscopy and low-temperature scanning electron microscopy. Choline residues of cell wall teichoic acids were found to play a fundamental role in pneumococcal biofilm development. The role in biofilm formation of choline-binding proteins, which anchor to the teichoic acids of the cell envelope, was determined using unambiguously characterized mutants. The results showed that LytA amidase, LytC lysozyme, LytB glucosaminidase, CbpA adhesin, PcpA putative adhesin, and PspA (pneumococcal surface protein A) mutants had a decreased capacity to form biofilms, whereas no such reduction was observed in Pce phosphocholinesterase or CbpD putative amidase mutants. Moreover, encapsulated, clinical pneumococcal isolates were impaired in their capacity to form biofilms. In addition, a role for extracellular DNA and proteins in the establishment of S. pneumoniae biofilms was demonstrated. Taken together, these observations provide information on conditions that favor the sessile mode of growth by S. pneumoniae. The experimental approach described here should facilitate the study of bacterial genes that are required for biofilm formation. Those results, in turn, may provide insight into strategies to prevent pneumococcal colonization of its human host.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

