Decreased expression of Bmi1 is closely associated with cellular senescence in small bile ducts in primary biliary cirrhosis
- PMID: 16936260
- PMCID: PMC1698839
- DOI: 10.2353/ajpath.2006.051237
Decreased expression of Bmi1 is closely associated with cellular senescence in small bile ducts in primary biliary cirrhosis
Abstract
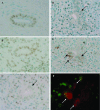
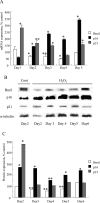
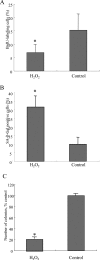
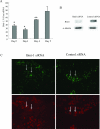
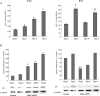
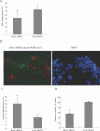
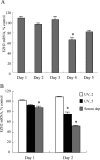
Cellular senescence of biliary epithelial cells with p16INK4a and p21WAF1/Cip expression in damaged small bile ducts may be critical for progressive bile duct loss in primary biliary cirrhosis. We investigated the involvement of bmi1, a polycomb group gene repressing p16INK4a expression, in the pathogenesis of biliary cellular senescence. Bmi1 expression was examined immunohistochemically in livers taken from the patients with primary biliary cirrhosis (n=18) and other diseased (n=19) and normal livers (n=16). We examined the effect of oxidative stress and a short interference RNA (siRNA) targeting bmi1 on cellular senescence in cultured mouse biliary epithelial cells. Bmi1 was widely expressed in the nuclei of biliary epithelial cells in the control livers. In contrast, bmi1 expression was significantly decreased in damaged small bile ducts in 43% of livers with primary biliary cirrhosis of stage 1/2, coordinating with the increased p16INK4a expression. In cultured biliary epithelial cells, oxidative stress by H2O2 treatment significantly decreased bmi1 expression, followed by increased P16INK4a expression. A knockdown of bmi1 induced increased p16INK4a expression, decreased cell proliferation, and increased cellular senescence. In conclusion, the decreased bmi1 expression caused by oxidative stress may be involved in the pathogenesis of cellular senescence of biliary epithelial cells in primary biliary cirrhosis.
Figures
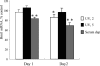
Similar articles
-
Frequent cellular senescence in small bile ducts in primary biliary cirrhosis: a possible role in bile duct loss.J Pathol. 2005 Mar;205(4):451-9. doi: 10.1002/path.1729. J Pathol. 2005. PMID: 15685690
-
Telomere shortening in the damaged small bile ducts in primary biliary cirrhosis reflects ongoing cellular senescence.Hepatology. 2008 Jul;48(1):186-95. doi: 10.1002/hep.22348. Hepatology. 2008. PMID: 18536059
-
Polycomb group protein Bmi1 is overexpressed and essential in anchorage-independent colony formation, cell proliferation and repression of cellular senescence in cholangiocarcinoma: tissue and culture studies.Hum Pathol. 2009 Dec;40(12):1723-30. doi: 10.1016/j.humpath.2009.01.027. Epub 2009 Aug 19. Hum Pathol. 2009. PMID: 19695678
-
Bmi1, stem cells, and senescence regulation.J Clin Invest. 2004 Jan;113(2):175-9. doi: 10.1172/JCI20800. J Clin Invest. 2004. PMID: 14722607 Free PMC article. Review.
-
Molecular mechanisms of cholangiopathy in primary biliary cirrhosis.Med Mol Morphol. 2006 Jun;39(2):55-61. doi: 10.1007/s00795-006-0321-z. Med Mol Morphol. 2006. PMID: 16821141 Review.
Cited by
-
Biliary Epithelial Senescence in Liver Disease: There Will Be SASP.Front Mol Biosci. 2021 Dec 21;8:803098. doi: 10.3389/fmolb.2021.803098. eCollection 2021. Front Mol Biosci. 2021. PMID: 34993234 Free PMC article. Review.
-
Tutorial review for understanding of cholangiopathy.Int J Hepatol. 2012;2012:547840. doi: 10.1155/2012/547840. Epub 2011 Aug 17. Int J Hepatol. 2012. PMID: 21994886 Free PMC article.
-
The dynamic biliary epithelia: molecules, pathways, and disease.J Hepatol. 2013 Mar;58(3):575-82. doi: 10.1016/j.jhep.2012.10.011. Epub 2012 Oct 17. J Hepatol. 2013. PMID: 23085249 Free PMC article. Review.
-
Interferon-induced protein with tetratricopeptide repeats 3 may be a key factor in primary biliary cholangitis.Sci Rep. 2021 Jun 1;11(1):11413. doi: 10.1038/s41598-021-91016-6. Sci Rep. 2021. PMID: 34075171 Free PMC article.
-
Novel approach to bile duct damage in primary biliary cirrhosis: participation of cellular senescence and autophagy.Int J Hepatol. 2012;2012:452143. doi: 10.1155/2012/452143. Epub 2011 Jul 7. Int J Hepatol. 2012. PMID: 21994884 Free PMC article.
References
-
- Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. - PubMed
-
- Kaplan M. Primary biliary cirrhosis. N Engl J Med. 1996;335:1570–1580. - PubMed
-
- Portmann B, Nakanuma Y. MacSween R, Burt A, Portmann B, Ishak K, Scheuer P, Anthony P, editors. London: Churchill Livingstone,; Diseases of the bile ducts. Pathology of the Liver, (ed 4) 2001:pp 435–506.
-
- Nakanuma Y, Ohta G. Histometric and serial section observations of the intrahepatic bile ducts in primary biliary cirrhosis. Gastroenterology. 1979;76:1326–1332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

