MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology
- PMID: 16936636
- PMCID: PMC1618377
- DOI: 10.1038/sj.embor.7400790
MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology
Abstract
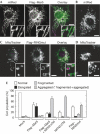
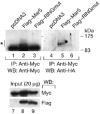
Mitofusins and Drp1 are key components in mitochondrial membrane fusion and division, but the molecular mechanism underlying the regulation of their activities remains to be clarified. Here, we identified human membrane-associated RING-CH (MARCH)-V as a novel transmembrane protein of the mitochondrial outer membrane. Immunoprecipitation studies demonstrated that MARCH-V interacts with mitofusin 2 (MFN2) and ubiquitinated forms of Drp1. Overexpression of MARCH-V promoted the formation of long tubular mitochondria in a manner that depends on MFN2 activity. By contrast, mutations in the RING finger caused fragmentation of mitochondria. We also show that MARCH-V promotes ubiquitination of Drp1. These results indicate that MARCH-V has a crucial role in the control of mitochondrial morphology by regulating MFN2 and Drp1 activities.
Figures


References
-
- Eura Y, Ishihara N, Yokota S, Mihara K (2003) Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem (Tokyo) 134: 333–344 - PubMed
-
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

