Retinoblastoma family genes
- PMID: 16936737
- PMCID: PMC1899835
- DOI: 10.1038/sj.onc.1209651
Retinoblastoma family genes
Abstract
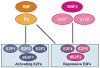
The retinoblastoma gene Rb was the first tumor suppressor gene cloned, and it is well known as a negative regulator of the cell cycle through its ability to bind the transcription factor E2F and repress transcription of genes required for S phase. Although over 100 other proteins have been reported to interact with Rb, in most cases these interactions are much less well characterized. Therefore, this review will primarily focus on Rb and E2F interactions. In addition to cell cycle regulation, studies of Rb and E2F proteins in animal models have revealed important roles for these proteins in apoptosis and differentiation. Recent screens of Rb/E2F target genes have identified new targets in all these areas. In addition, the mechanisms determining how different subsets of target genes are regulated under different conditions have only begun to be addressed and offer exciting possibilities for future research.
Figures



References
-
- Apostolova MD, Ivanova IA, Dagnino C, D’Souza SJ, Dagnino L. J Biol Chem. 2002;277:34471–34479. - PubMed
-
- Asano M, Nevins JR, Wharton RP. Genes Dev. 1996;10:1422–1432. - PubMed
-
- Attwooll C, Oddi S, Cartwright P, Prosperini E, Agger K, Steensgaard P, et al. J Biol Chem. 2004b;280:1199–1208. - PubMed
-
- Bosco G, Du W, Orr-Weaver TL. Nat Cell Biol. 2001;3:289–295. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

