Effects of chronic therapy with non-steroidal antiinflammatory drugs on gastric permeability of sucrose: a study on 71 patients with rheumatoid arthritis
- PMID: 16937498
- PMCID: PMC4087405
- DOI: 10.3748/wjg.v12.i31.5017
Effects of chronic therapy with non-steroidal antiinflammatory drugs on gastric permeability of sucrose: a study on 71 patients with rheumatoid arthritis
Abstract
Aim: To evaluate the gastric permeability after both acute and chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) and to assess the clinical usefulness of sucrose test in detecting and following NSAIDs- induced gastric damage mainly in asymptomatic patients and the efficacy of a single pantoprazole dose in chronic users.
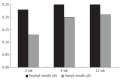
Methods: Seventy-one consecutive patients on chronic therapy with NSAIDs were enrolled in the study and divided into groups A and B (group A receiving 40 mg pantoprazole daily, group B only receiving NSAIDs). Sucrose test was performed at baseline and after 2, 4 and 12 wk, respectively. The symptoms in the upper gastrointestinal tract were recorded.
Results: The patients treated with pantoprazole had sucrose excretion under the limit during the entire follow-up period. The patients without gastroprotection had sucrose excretion above the limit after 2 wk, with an increasing trend in the following weeks (P = 0.000). A number of patients in this group revealed a significantly altered gastric permeability although they were asymptomatic during the follow-up period.
Conclusion: Sucrose test can be proposed as a valid tool for the clinical evaluation of NSAIDs- induced gastric damage in both acute and chronic therapy. This technique helps to identify patients with clinically silent gastric damages. Pantoprazole (40 mg daily) is effective and well tolerated in chronic NSAID users.
Figures
Similar articles
-
Efficacy and tolerability of pantoprazole compared with misoprostol for the prevention of NSAID-related gastrointestinal lesions and symptoms in rheumatic patients.Digestion. 2003;68(4):198-208. doi: 10.1159/000075926. Epub 2003 Dec 30. Digestion. 2003. PMID: 14707396 Clinical Trial.
-
Mechanisms of protection by pantoprazole against NSAID-induced gastric mucosal damage.Naunyn Schmiedebergs Arch Pharmacol. 2005 Jul;372(1):79-87. doi: 10.1007/s00210-005-1075-1. Epub 2005 Aug 4. Naunyn Schmiedebergs Arch Pharmacol. 2005. PMID: 16080005
-
Intragastric acid control in non-steroidal anti-inflammatory drug users: comparison of esomeprazole, lansoprazole and pantoprazole.Aliment Pharmacol Ther. 2006 Apr 15;23(8):1189-96. doi: 10.1111/j.1365-2036.2006.02867.x. Aliment Pharmacol Ther. 2006. PMID: 16611280 Clinical Trial.
-
Nonsteroidal anti-inflammatory drugs: add an anti-ulcer drug for patients at high risk only. Always limit the dose and duration of treatment with NSAIDs.Prescrire Int. 2011 Sep;20(119):216-9. Prescrire Int. 2011. PMID: 21954519 Review.
-
Pantoprazole: an update of its pharmacological properties and therapeutic use in the management of acid-related disorders.Drugs. 2003;63(1):101-33. doi: 10.2165/00003495-200363010-00006. Drugs. 2003. PMID: 12487624 Review.
Cited by
-
Influence of prolonged exposure of a short half life non-steroidal anti-inflammatory drugs on gastrointestinal safety.Inflammopharmacology. 2009 Aug;17(4):205-10. doi: 10.1007/s10787-009-0007-y. Epub 2009 Jul 8. Inflammopharmacology. 2009. PMID: 19585082
References
-
- Retail & Provider Perspective, National Prescription Audit, 1999-2000. Plymouth, PA: IMS Health; 2000.
-
- Editoriale: Il mercato farmaceutico mondiale nel 2001. Bollettino Informazione sui farmaci. 2001;6:275–277.
-
- Koutsos MI, Shiff SJ, Rigas B. Can nonsteroidal anti-inflammatory drugs be recommended to prevent colon cancer in high risk elderly patients. Drugs Aging. 1995;6:421–425. - PubMed
-
- Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. - PubMed
-
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical