The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors
- PMID: 16938849
- PMCID: PMC1569174
- DOI: 10.1073/pnas.0605950103
The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors
Abstract
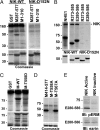
The mammalian Ste20-like Nck-interacting kinase (NIK) and its orthologs Misshapen in Drosophila and Mig-15 in Caenorhabditis elegans have a conserved function in regulating cell morphology, although through poorly understood mechanisms. We report two previously unrecognized actions of NIK: regulation of lamellipodium formation by growth factors and phosphorylation of the ERM proteins ezrin, radixin, and moesin. ERM proteins regulate cell morphology and plasma membrane dynamics by reversibly anchoring actin filaments to integral plasma membrane proteins. In vitro assays show that NIK interacts directly with ERM proteins, binding their N termini and phosphorylating a conserved C-terminal threonine. In cells, NIK and phosphorylated ERM proteins localize at the distal margins of lamellipodia, and NIK activity is necessary for phosphorylation of ERM proteins induced by EGF and PDGF, but not by thrombin. Lamellipodium extension in response to growth factors is inhibited in cells expressing a kinase-inactive NIK, suppressed for NIK expression with siRNA oligonucleotides, or expressing ezrin T567A that cannot be phosphorylated. These data suggest that direct phosphorylation of ERM proteins by NIK constitutes a signaling mechanism controlling growth factor-induced membrane protrusion and cell morphology.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
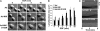




References
-
- Fu C. A., Shen M., Huang B. C., Lasaga J., Payan D. G., Luo Y. J. Biol. Chem. 1999;274:30729–30737. - PubMed
-
- Hu Y., Leo C., Yu S., Huang B. C., Wang H., Shen M., Luo Y., Daniel-Issakani S., Payan D. G., Xu X. J. Biol. Chem. 2004;279:54387–54397. - PubMed
-
- Nakano K., Kanai-Azuma M., Kanai Y., Moriyama K., Yazaki K., Hayashi Y., Kitamura N. Exp. Cell Res. 2003;287:219–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

