Polyvalent inhibitors of anthrax toxin that target host receptors
- PMID: 16938891
- PMCID: PMC1569193
- DOI: 10.1073/pnas.0509870103
Polyvalent inhibitors of anthrax toxin that target host receptors
Abstract
Resistance of pathogens to antimicrobial therapeutics has become a widespread problem. Resistance can emerge naturally, but it can also be engineered intentionally, which is an important consideration in designing therapeutics for bioterrorism agents. Blocking host receptors used by pathogens represents a powerful strategy to overcome this problem, because extensive alterations to the pathogen may be required to enable it to switch to a new receptor that can still support pathogenesis. Here, we demonstrate a facile method for producing potent receptor-directed antitoxins. We used phage display to identify a peptide that binds both anthrax-toxin receptors and attached this peptide to a synthetic scaffold. Polyvalency increased the potency of these peptides by >50,000-fold in vitro and enabled the neutralization of anthrax toxin in vivo. This work demonstrates a receptor-directed anthrax-toxin inhibitor and represents a promising strategy to combat a variety of viral and bacterial diseases.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
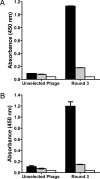
References
-
- Levy S. B., Marshall B. Nat. Med. 2004;10:S122–S129. - PubMed
-
- Hogan D., Kolter R. Curr. Opin. Microbiol. 2002;5:472–477. - PubMed
-
- Mammen M., Choi S. K., Whitesides G. M. Angew. Chem. Int. Ed. 1998;37:2754–2794. - PubMed
-
- Mammen M., Dahmann G., Whitesides G. M. J. Med. Chem. 1995;38:4179–4190. - PubMed
-
- Mochalova L. V., Tuzikov A. B., Marinina V. P., Gambaryan A. S., Byramova N. E., Bovin N. V., Matrosovich M. N. Antiviral Res. 1994;23:179–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

