Dose selection and population pharmacokinetics of PEG-Intron in patients with chronic myelogenous leukaemia
- PMID: 16939523
- PMCID: PMC2000735
- DOI: 10.1111/j.1365-2125.2006.02757.x
Dose selection and population pharmacokinetics of PEG-Intron in patients with chronic myelogenous leukaemia
Abstract
Aims: To assess the dose selection using population pharmacokinetics of Pegylated Intron-alpha2b (PEG-Intron) in patients with chronic myelogenous leukaemia (CML).
Methods: PEG-Intron 3-6 microg kg(-1) was administered subcutaneously once a week and blood samples were collected up to 48 weeks of treatment. A total of 624 samples collected from 137 patients were included in the analysis. Nonlinear mixed-effects modelling was used to analyse the sparsely sampled concentration data from a clinical efficacy trial. Covariates in the analysis included weight, sex, age, race, serum creatinine and estimated creatinine clearance (CLcr).
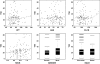
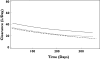
Results: The apparent clearance of PEG-Intron decreased after repeated dosing. The clearance at treatment week 4 was 42.3 l day(-1) (patients with CLcr 120 ml min(-1)) with interpatient variability 30%. At treatment week 48, the clearance value was reduced to 69% of its week 4 value. CLcr, a composite variable calculated from body weight, sex, age and serum creatinine, had a small but statistically significant influence on the clearance of PEG-Intron. The clearance of PEG-Intron in patients with CML was 40% higher than that of hepatitis C virus-infected patients.
Conclusion: The dose of PEG-Intron 6.0 microg kg(-1) week(-1) appeared appropriate in the treatment of patients with CML.
Figures


Similar articles
-
Treating cancer with PEG Intron: pharmacokinetic profile and dosing guidelines for an improved interferon-alpha-2b formulation.Cancer. 2002 Jul 15;95(2):389-96. doi: 10.1002/cncr.10663. Cancer. 2002. PMID: 12124839 Review.
-
Phase I evaluation of a 40-kDa branched-chain long-acting pegylated IFN-alpha-2a with and without cytarabine in patients with chronic myelogenous leukemia.Clin Cancer Res. 2005 Sep 1;11(17):6247-55. doi: 10.1158/1078-0432.CCR-05-0882. Clin Cancer Res. 2005. PMID: 16144928 Clinical Trial.
-
Pegylated interferon-alpha2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Hepatitis C Intervention Therapy Group.Clin Pharmacol Ther. 2000 Nov;68(5):556-67. doi: 10.1067/mcp.2000.110973. Clin Pharmacol Ther. 2000. PMID: 11103758 Clinical Trial.
-
Single-dose pharmacokinetics and safety of pegylated interferon-alpha2b in patients with chronic renal dysfunction.J Clin Pharmacol. 2002 Oct;42(10):1109-15. doi: 10.1177/009127002401382713. J Clin Pharmacol. 2002. PMID: 12362925 Clinical Trial.
-
A dose-ranging study of pegylated interferon alfa-2b and ribavirin in chronic hepatitis C. The Hepatitis C Intervention Therapy Group.Hepatology. 2000 Sep;32(3):647-53. doi: 10.1053/jhep.2000.16661. Hepatology. 2000. PMID: 10960463 Clinical Trial.
Cited by
-
Multimeric peptide-based PEG nanocarriers with programmable elimination properties.Biomaterials. 2009 Oct;30(29):5649-59. doi: 10.1016/j.biomaterials.2009.05.068. Epub 2009 Jul 31. Biomaterials. 2009. PMID: 19647312 Free PMC article.
References
-
- Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia. Biol Ther Ann Intern Med. 1999;131:207. - PubMed
-
- Kantarjian HM, O’Brien S, Anderlini P, Talpaz M. Treatment of chronic myelogenous leukemia: current status and investigational options. Blood. 1996;87:3069–81. - PubMed
-
- Talpaz M, Kantarjian H, Kurzrock R. Interferon-alpha produces sustained cytogenetic responses in chronic myelogenous leukemia. Ann Intern Med. 1991;114:532. - PubMed
-
- Kantarjian HM, Smith TL, O’Brien S. Prolonged survival in chronic myelogenous leukemia after cytogenetic response to interferon-α therapy. Ann Intern Med. 1995;122:254–61. - PubMed
-
- Glue P, Fang JW, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, Jacobs S and the Hepatitis C Study Group. PEG-interferon alfa-2b: pharmacokinetics, pharmacodynamics, safety and preliminary efficacy data. Clin Pharmacol Ther. 2000;68:556–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

