Cell replacement therapy in neurological disease
- PMID: 16939969
- PMCID: PMC1664668
- DOI: 10.1098/rstb.2006.1886
Cell replacement therapy in neurological disease
Abstract
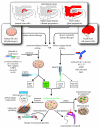
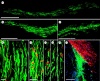
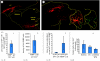
Diseases of the brain and spinal cord represent especially daunting challenges for cell-based strategies of repair, given the multiplicity of cell types within the adult central nervous system, and the precision with which they must interact in both space and time. Nonetheless, a number of diseases are especially appropriate for cell-based therapy, in particular those in which single phenotypes are lost, and in which the re-establishment of vectorially specific connections is not entirely requisite for therapeutic benefit. We review here a set of potential therapeutic indications that meet these criteria as potentially benefiting from the transplantation of neural stem and progenitor cells. These include: (i) transplantation of phenotypically restricted neuronal progenitor cells into diseases of a single neuronal phenotype, such as Parkinson's disease; (ii) implantation of mixed progenitor pools into diseases characterized by the loss of a limited number of discrete phenotypes, such as spinal cord injury and the motor neuronopathies; (iii) transplantation of glial and nominally oligodendrocytic progenitor cells as a means of treating disorders of myelin; and (iv) transplantation of neural stem cells as a means of treating lysosomal storage disorders and other diseases of enzymatic deficiency. Among the diseases potentially approachable by these strategies, the myelin disorders, including the paediatric leucodystrophies as well as adult traumatic and inflammatory demyelinations, may present the most compelling targets for cell-based neurological therapy.
Figures
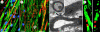

References
-
- Brustle O, Choudhary K, Karram K, Huttner A, Murray K, Dubois-Dalcq M, McKay R. Chimeric brains generated by intraventricular transplantation of fetal human brain cells into embryonic rats. Nat. Biotechnol. 1998;16:1040–1044. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
