Intracellular control of developmental and regenerative axon growth
- PMID: 16939976
- PMCID: PMC1664665
- DOI: 10.1098/rstb.2006.1882
Intracellular control of developmental and regenerative axon growth
Abstract
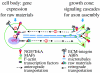
Axon growth is a highly regulated process that requires stimulating signals from extracellular factors. The extracellular signals are then transduced to regulate coordinately gene expression and local axon assembly. Growth factors, especially neurotrophins that act via receptor tyrosine kinases, have been heavily studied as extracellular factors that stimulate axon growth. Downstream of receptor tyrosine kinases, recent studies have suggested that phosphatidylinositol-3 kinase (PI3K) regulates local assembly of axonal cytoskeleton, especially microtubules, via glycogen synthase kinase 3beta (GSK-3beta) and multiple microtubule binding proteins. The role of extracellular signal regulated kinase (ERK) signalling in regulation of local axon assembly is less clear, but may involve the regulation of local protein translation. Gene expression during axon growth is regulated by transcription factors, among which cyclic AMP response element binding protein and nuclear factors of activated T-cells (NFATs) are known to be required for neurotrophin (NT)-induced axon extension. In addition to growth factors, extracellular matrix molecules and neuronal activity contribute importantly to control axon growth. Increasingly, evidence suggests that these influences act to enhance growth via coordinating with growth factor signalling. Finally, evidence is emerging that developmental versus regenerative axon growth may be mediated by distinct signalling pathways, both at the level of gene transcription and at the level of local axon assembly.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
