Population pharmacokinetics of tigecycline in patients with complicated intra-abdominal or skin and skin structure infections
- PMID: 16940069
- PMCID: PMC1635236
- DOI: 10.1128/AAC.01636-05
Population pharmacokinetics of tigecycline in patients with complicated intra-abdominal or skin and skin structure infections
Abstract
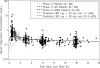
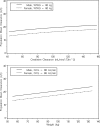
Tigecycline, a first-in-class expanded glycylcycline antimicrobial agent, has demonstrated efficacy in the treatment of complicated skin and skin structure infections (cSSSI) and complicated intra-abdominal (cIAI) infections. A population pharmacokinetic (PK) model for tigecycline was developed for patients with cSSSI or cIAI enrolled in two phase 2 clinical trials, and the influence of selected demographic factors and clinical laboratory measures was investigated. Tigecycline was administered as an intravenous loading dose followed by a 0.5- or 1-h infusion every 12 h for up to 14 days. Blood samples were collected the day before or the day of hospital discharge for the determination of serum tigecycline concentrations. Patient covariates were evaluated using stepwise forward (alpha = 0.05) and backward (alpha = 0.001) procedures. The predictive performance of the model was assessed separately using pooled data from either two phase 3 studies for patients with cSSSI or two phase 3 studies for patients with cIAI. A two-compartment model with zero-order input and first-order elimination adequately described the steady-state tigecycline concentration-time data. Tigecycline clearance was shown to increase with increasing weight, increasing creatinine clearance, and male gender (P < 0.001). The final model provided a relatively unbiased fit to each data set. Individual predicted values of the area under the concentration-time curve from 0 to 12 h (AUC(0-12)) were generally unbiased (median prediction error, -1.60% to -3.78%) and were similarly precise (median absolute prediction error, <4%) when compared across data sets. The population PK model provided the basis to obtain individual estimates of steady-state AUC(0-12) in later exposure-response analyses of tigecycline safety and efficacy in patients with cSSSI or cIAI.
Figures

Similar articles
-
Population pharmacokinetics of tigecycline in healthy volunteers.J Clin Pharmacol. 2007 Jun;47(6):727-37. doi: 10.1177/0091270007300263. J Clin Pharmacol. 2007. PMID: 17519399
-
Pharmacokinetics and safety profile of tigecycline in children aged 8 to 11 years with selected serious infections: a multicenter, open-label, ascending-dose study.Clin Ther. 2012 Feb;34(2):496-507.e1. doi: 10.1016/j.clinthera.2011.12.010. Epub 2012 Jan 16. Clin Ther. 2012. PMID: 22249106 Clinical Trial.
-
The pharmacokinetic and pharmacodynamic profile of tigecycline.Clin Infect Dis. 2005 Sep 1;41 Suppl 5:S333-40. doi: 10.1086/431674. Clin Infect Dis. 2005. PMID: 16080071 Review.
-
Exposure-response analyses of tigecycline efficacy in patients with complicated skin and skin-structure infections.Antimicrob Agents Chemother. 2007 Jun;51(6):1939-45. doi: 10.1128/AAC.01084-06. Epub 2007 Mar 12. Antimicrob Agents Chemother. 2007. PMID: 17353238 Free PMC article. Clinical Trial.
-
Pharmacokinetic/pharmacodynamic profile for tigecycline-a new glycylcycline antimicrobial agent.Diagn Microbiol Infect Dis. 2005 Jul;52(3):165-71. doi: 10.1016/j.diagmicrobio.2005.05.006. Diagn Microbiol Infect Dis. 2005. PMID: 16105560 Review.
Cited by
-
In vitro pharmacodynamics of simulated pulmonary exposures of tigecycline alone and in combination against Klebsiella pneumoniae isolates producing a KPC carbapenemase.Antimicrob Agents Chemother. 2011 Apr;55(4):1420-7. doi: 10.1128/AAC.01253-10. Epub 2011 Jan 31. Antimicrob Agents Chemother. 2011. PMID: 21282442 Free PMC article.
-
Nonlinear Protein Binding: Not What You Think.J Pharm Sci. 2018 Jul;107(7):1754-1760. doi: 10.1016/j.xphs.2018.03.023. Epub 2018 Apr 4. J Pharm Sci. 2018. PMID: 29626534 Free PMC article. Review.
-
Tissue Penetration of Antimicrobials in Intensive Care Unit Patients: A Systematic Review-Part II.Antibiotics (Basel). 2022 Sep 3;11(9):1193. doi: 10.3390/antibiotics11091193. Antibiotics (Basel). 2022. PMID: 36139972 Free PMC article. Review.
-
Evaluation of the stability of tigecycline in elastomeric infusion devices used for outpatient parenteral antimicrobial therapy.JAC Antimicrob Resist. 2025 May 13;7(3):dlaf074. doi: 10.1093/jacamr/dlaf074. eCollection 2025 Jun. JAC Antimicrob Resist. 2025. PMID: 40365447 Free PMC article.
-
Evaluation of tigecycline penetration into colon wall tissue and epithelial lining fluid using a population pharmacokinetic model and Monte Carlo simulation.Antimicrob Agents Chemother. 2007 Nov;51(11):4085-9. doi: 10.1128/AAC.00065-07. Epub 2007 Sep 10. Antimicrob Agents Chemother. 2007. PMID: 17846139 Free PMC article. Clinical Trial.
References
-
- Babinchak, T., E. J. Ellis-Grosse, N. Dartois, G. M. Rose, and E. Loh. 2005. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trials data. Clin. Infect. Dis. 41(Suppl. 5):S354-S367. - PubMed
-
- Beal, S. L., and L. B. Sheiner (ed.). 1992. NONMEM users guides. GloboMax, LLC, Hanover, Md.
-
- Bradford, P. A. 2004. Tigecycline: a first in class glycylcycline. Clin. Microbiol. Newsl. 26:163-168.
-
- Conte, J. E., J. A. Golden, M. G. Kelly, and E. Zurlinden. 2005. Steady-state serum and intrapulmonary pharmacokinetics and pharmacodynamics of tigecycline. Int. J. Antimicrob. Agents 25:523-529. - PubMed
-
- Ellis-Grosse, E. J., T. Babinchak, N. Dartois, G. Rose, and E. Loh. 2005. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin. Infect. Dis. 41(Suppl. 5):S341-S353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

