Host-derived pentapeptide affecting adhesion, proliferation, and local pH in biofilm communities composed of Streptococcus and Actinomyces species
- PMID: 16940141
- PMCID: PMC1695511
- DOI: 10.1128/IAI.00068-06
Host-derived pentapeptide affecting adhesion, proliferation, and local pH in biofilm communities composed of Streptococcus and Actinomyces species
Abstract
Salivary proline-rich proteins (PRPs) attach commensal Actinomyces and Streptococcus species to teeth. Here, gel filtration, mass spectrometry and Edman degradation were applied to show the release of a pentapeptide, RGRPQ, from PRP-1 upon proteolysis by Streptococcus gordonii. Moreover, synthetic RGRPQ and derivatives were used to investigate associated innate properties and responsible motifs. The RGRPQ peptide increased 2.5-fold the growth rate of S. gordonii via a Q-dependent sequence motif and selectively stimulated oral colonization of this organism in a rat model in vivo. In contrast, the growth of Streptococcus mutans, implicated in caries, was not affected. While the entire RGRPQ sequence was required to block sucrose-induced pH-decrease by S. gordonii and S. mutans, the N-terminal Arg residue mediated the pH increase (i.e., ammonia production) by S. gordonii alone (which exhibits Arg catabolism to ammonia). Strains of commensal viridans streptococci exhibited PRP degradation and Arg catabolism, whereas cariogenic species did not. The RGRPQ peptide mediated via a differential Q-dependent sequence motif, adhesion inhibition, and desorption of PRP-1-binding strains of A. naeslundii genospecies 2 (5 of 10 strains) but not of S. gordonii (n=5). The inhibitable A. naeslundii strains alone displayed the same binding profile as S. gordonii to hybrid peptides terminating in RGRPQ or GQSPQ, derived from the middle or C-terminal segments of PRP-1. The present findings indicate the presence of a host-bacterium interaction in which a host peptide released by bacterial proteolysis affects key properties in biofilm formation.
Figures
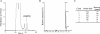
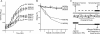
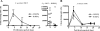
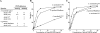
References
-
- Ayad, M., B. C. Van Wuyckhuyse, K. Minaguchi, R. F. Raubertas, G. S. Bedi, R. J. Billings, W. H. Bowen, and L. A. Tabak. 2000. The association of basic proline-rich peptides from human parotid gland secretions with caries experience. J. Dent. Res. 79:976-982. - PubMed
-
- Azen, E. A., and N. Maeda. 1988. Molecular genetics of human salivary proteins and their polymorphisms. Adv. Hum. Genet. 17:141-199. - PubMed
-
- Björck, L., P. Åkesson, M. Bohus, J. Trojnar, M. Abrahamson, I. Olafsson, and A. Grubb. 1989. Bacterial growth blocked by a synthetic peptide based on the structure of a human proteinase inhibitor. Nature 337:385-386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

