The plasminogen activator system modulates sympathetic nerve function
- PMID: 16940168
- PMCID: PMC2118409
- DOI: 10.1084/jem.20060077
The plasminogen activator system modulates sympathetic nerve function
Abstract
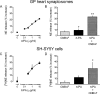
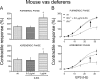
Sympathetic neurons synthesize and release tissue plasminogen activator (t-PA). We investigated whether t-PA modulates sympathetic activity. t-PA inhibition markedly reduced contraction of the guinea pig vas deferens to electrical field stimulation (EFS) and norepinephrine (NE) exocytosis from cardiac synaptosomes. Recombinant t-PA (rt-PA) induced exocytotic and carrier-mediated NE release from cardiac synaptosomes and cultured neuroblastoma cells; this was a plasmin-independent effect but was potentiated by a fibrinogen cleavage product. Notably, hearts from t-PA-null mice released much less NE upon EFS than their wild-type (WT) controls (i.e., a 76.5% decrease; P<0.01), whereas hearts from plasminogen activator inhibitor-1 (PAI-1)-null mice released much more NE (i.e., a 275% increase; P<0.05). Furthermore, vasa deferentia from t-PA-null mice were hyporesponsive to EFS (P<0.0001) but were normalized by the addition of rt-PA. In contrast, vasa from PAI-1-null mice were much more responsive (P<0.05). Coronary NE overflow from hearts subjected to ischemia/reperfusion was much smaller in t-PA-null than in WT control mice (P<0.01). Furthermore, reperfusion arrhythmias were significantly reduced (P<0.05) in t-PA-null hearts. Thus, t-PA enhances NE release from sympathetic nerves and contributes to cardiac arrhythmias in ischemia/reperfusion. Because the risk of arrhythmias and sudden cardiac death is increased in hyperadrenergic conditions, targeting the NE-releasing effect of t-PA may have valuable therapeutic potential.
Figures

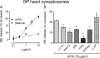
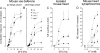


References
-
- Eijnden-Schrauwen, Y., T. Kooistra, R. E. de Vries, and J.J. Emeis. 1995. Studies on the acute release of tissue-type plasminogen activator from human endothelial cells in vitro and in rats in vivo: evidence for a dynamic storage pool. Blood. 85:3510–3517. - PubMed
-
- Wang, Y., X. Jiang, A.R. Hand, C. Gilles, J. Kirk, R.E. Cone, and J. O'Rourke. 2002. Additional evidence that the sympathetic nervous system regulates the vessel wall release of tissue plasminogen activator. Blood Coagul. Fibrinolysis. 13:471–481. - PubMed
-
- O'Rourke, J., X. Jiang, Z. Hao, R.E. Cone, and A.R. Hand. 2005. Distribution of sympathetic tissue plasminogen activator (tPA) to a distant microvasculature. J. Neurosci. Res. 79:727–733. - PubMed
-
- Collen, D. 1999. The plasminogen (fibrinolytic) system. Thromb. Haemost. 82:259–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

