Distinct signaling pathways in TRAIL- versus tumor necrosis factor-induced apoptosis
- PMID: 16940186
- PMCID: PMC1636728
- DOI: 10.1128/MCB.00257-06
Distinct signaling pathways in TRAIL- versus tumor necrosis factor-induced apoptosis
Abstract
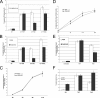
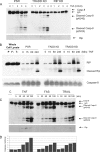
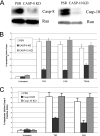
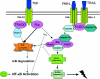
Trimeric tumor necrosis factor (TNF) binding leads to recruitment of TRADD to TNFR1. In current models, TRADD recruits RIP, TRAF2, and FADD to activate NF-kappaB, Jun N-terminal protein kinase (JNK), and apoptosis. Using stable short-hairpin RNA (shRNA) knockdown (KD) cells targeting these adaptors, TNF death-inducing signaling complex immunoprecipitation demonstrates competitive binding of TRADD and RIP to TNFR1, whereas TRAF2 recruitment requires TRADD. Analysis of KD cells indicates that FADD is necessary for Fas-L- or TRAIL- but not TNF-induced apoptosis. Interestingly, TRADD is dispensable, while RIP is required for TNF-induced apoptosis in human tumor cells. TRADD is required for c-Jun phosphorylation upon TNF exposure. RIP KD abrogates formation of complex II following TNF exposure, whereas TRADD KD allows efficient RIP-caspase 8 association. Treatment with TRAIL also induces formation of a complex II containing FADD, RIP, IKKalpha, and caspase 8 and 10, leading to activation of caspase 8. Our data suggest that TNF triggers apoptosis in a manner distinct from that of Fas-L or TRAIL.
Figures



Similar articles
-
Fas-associated death domain protein and caspase-8 are not recruited to the tumor necrosis factor receptor 1 signaling complex during tumor necrosis factor-induced apoptosis.J Biol Chem. 2003 Jul 11;278(28):25534-41. doi: 10.1074/jbc.M303399200. Epub 2003 Apr 29. J Biol Chem. 2003. PMID: 12721308
-
Mechanism of chronic obstructive uropathy: increased expression of apoptosis-promoting molecules.Kidney Int. 2000 Oct;58(4):1481-91. doi: 10.1046/j.1523-1755.2000.00310.x. Kidney Int. 2000. PMID: 11012883
-
Stat1 as a component of tumor necrosis factor alpha receptor 1-TRADD signaling complex to inhibit NF-kappaB activation.Mol Cell Biol. 2000 Jul;20(13):4505-12. doi: 10.1128/MCB.20.13.4505-4512.2000. Mol Cell Biol. 2000. PMID: 10848577 Free PMC article.
-
Structural determinants of DISC function: new insights into death receptor-mediated apoptosis signalling.Pharmacol Ther. 2013 Nov;140(2):186-99. doi: 10.1016/j.pharmthera.2013.06.009. Epub 2013 Jul 8. Pharmacol Ther. 2013. PMID: 23845861 Review.
-
Pursuing different 'TRADDes': TRADD signaling induced by TNF-receptor 1 and the Epstein-Barr virus oncoprotein LMP1.Biol Chem. 2008 Oct;389(10):1261-71. doi: 10.1515/BC.2008.144. Biol Chem. 2008. PMID: 18713013 Review.
Cited by
-
Molecular crosstalk between apoptosis, necroptosis, and survival signaling.Mol Cell Oncol. 2015 Apr 8;2(4):e975093. doi: 10.4161/23723556.2014.975093. eCollection 2015 Oct-Dec. Mol Cell Oncol. 2015. PMID: 27308513 Free PMC article. Review.
-
Necroptosis in inflammatory bowel disease and other intestinal diseases.World J Clin Cases. 2018 Nov 26;6(14):745-752. doi: 10.12998/wjcc.v6.i14.745. World J Clin Cases. 2018. PMID: 30510938 Free PMC article. Review.
-
The death domain kinase RIP1 links the immunoregulatory CD40 receptor to apoptotic signaling in carcinomas.J Cell Biol. 2011 Feb 7;192(3):391-9. doi: 10.1083/jcb.201003087. Epub 2011 Jan 31. J Cell Biol. 2011. PMID: 21282461 Free PMC article.
-
EGFR-targeted diphtheria toxin stimulates TRAIL killing of glioblastoma cells by depleting anti-apoptotic proteins.J Neurooncol. 2009 Nov;95(2):175-184. doi: 10.1007/s11060-009-9914-4. Epub 2009 May 17. J Neurooncol. 2009. PMID: 19449148 Free PMC article.
-
Reduced internalization of TNF-ɑ/TNFR1 down-regulates caspase dependent phagocytosis induced cell death (PICD) in neonatal monocytes.PLoS One. 2017 Aug 9;12(8):e0182415. doi: 10.1371/journal.pone.0182415. eCollection 2017. PLoS One. 2017. PMID: 28793310 Free PMC article.
References
-
- Aggarwal, B. B. 2003. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3:745-756. - PubMed
-
- Ashkenazi, A., and V. M. Dixit. 1999. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 11:255-260. - PubMed
-
- Boatright, K. M., M. Renatus, F. L. Scott, S. Sperandio, H. Shin, I. M. Pedersen, J. E. Ricci, W. A. Edris, D. P. Sutherlin, D. R. Green, and G. S. Salvesen. 2003. A unified model for apical caspase activation. Mol. Cell 11:529-541. - PubMed
-
- Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2:243-247. - PubMed
-
- Chen, G., and D. V. Goeddel. 2002. TNF-R1 signaling: a beautiful pathway. Science 296:1634-1635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
