Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells
- PMID: 16940423
- PMCID: PMC1785076
- DOI: 10.1182/blood-2006-03-007153
Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells
Abstract
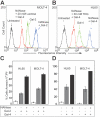
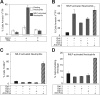
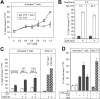
Cellular turnover is associated with exposure of surface phosphatidylserine (PS) in apoptotic cells, leading to their phagocytic recognition and removal. But recent studies indicate that surface PS exposure is not always associated with apoptosis. Here we show that several members of the human galectin family of glycan binding proteins (galectins-1, -2, and -4) induce PS exposure in a carbohydrate-dependent fashion in activated, but not resting, human neutrophils and in several leukocyte cell lines. PS exposure is not associated with apoptosis in activated neutrophils. The exposure of PS in cell lines treated with these galectins is sustained and does not affect cell viability. Unexpectedly, these galectins bind well to activated T lymphocytes, but do not induce either PS exposure or apoptosis, indicating that galectin's effects are cell specific. These results suggest novel immunoregulatory contribution of galectins in regulating leukocyte turnover independently of apoptosis.
Conflict of interest statement
Conflict of interest disclosure: The authors declare no competing financial interests.
Figures
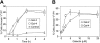



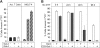
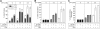
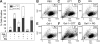
Similar articles
-
Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion.J Immunol. 2008 Mar 1;180(5):3091-102. doi: 10.4049/jimmunol.180.5.3091. J Immunol. 2008. PMID: 18292532
-
Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis.J Biol Chem. 2003 Oct 17;278(42):41282-93. doi: 10.1074/jbc.M306624200. Epub 2003 Jul 9. J Biol Chem. 2003. PMID: 12853445
-
Contributions of Ca2+ to galectin-1-induced exposure of phosphatidylserine on activated neutrophils.J Biol Chem. 2005 Aug 5;280(31):28623-31. doi: 10.1074/jbc.M414140200. Epub 2005 Jun 1. J Biol Chem. 2005. PMID: 15929990
-
Assembly, organization and regulation of cell-surface receptors by lectin-glycan complexes.Biochem J. 2015 Jul 1;469(1):1-16. doi: 10.1042/BJ20150461. Biochem J. 2015. PMID: 26173257 Review.
-
Binding and cross-linking properties of galectins.Biochim Biophys Acta. 2002 Sep 19;1572(2-3):255-62. doi: 10.1016/s0304-4165(02)00312-4. Biochim Biophys Acta. 2002. PMID: 12223273 Review.
Cited by
-
Synthetic glycoconjugates inhibitors of tumor-related galectin-3: an update.Glycoconj J. 2016 Dec;33(6):853-876. doi: 10.1007/s10719-016-9721-z. Epub 2016 Aug 15. Glycoconj J. 2016. PMID: 27526114 Review.
-
Galectin-1 signaling in leukocytes requires expression of complex-type N-glycans.Glycobiology. 2008 Oct;18(10):770-8. doi: 10.1093/glycob/cwn066. Epub 2008 Jul 16. Glycobiology. 2008. PMID: 18633135 Free PMC article.
-
Functions of cell surface galectin-glycoprotein lattices.Curr Opin Struct Biol. 2007 Oct;17(5):513-20. doi: 10.1016/j.sbi.2007.09.002. Epub 2007 Oct 22. Curr Opin Struct Biol. 2007. PMID: 17950594 Free PMC article. Review.
-
Galectins in hematological malignancies.Am J Blood Res. 2011;1(2):119-29. Epub 2011 Sep 7. Am J Blood Res. 2011. PMID: 22432074 Free PMC article.
-
Evaluation of the Bactericidal Activity of Galectins.Methods Mol Biol. 2022;2442:517-531. doi: 10.1007/978-1-0716-2055-7_27. Methods Mol Biol. 2022. PMID: 35320543
References
-
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. - PubMed
-
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. - PubMed
-
- Stroh C, Schulze-Osthoff K. Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 1998;5:997–1000. - PubMed
-
- Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. - PubMed
-
- Simon HU. Targeting apoptosis in the control of inflammation. Eur Respir J Suppl. 2003;44:20s–21s. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

