Maturation and enucleation of primitive erythroblasts during mouse embryogenesis is accompanied by changes in cell-surface antigen expression
- PMID: 16940424
- PMCID: PMC1785074
- DOI: 10.1182/blood-2006-03-006569
Maturation and enucleation of primitive erythroblasts during mouse embryogenesis is accompanied by changes in cell-surface antigen expression
Erratum in
- Blood. 2007 Jun 1;109(11):4616
Abstract
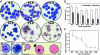
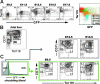
Primitive erythroblasts (EryPs) are the first hematopoietic cell type to form during mammalian embryogenesis and emerge within the blood islands of the yolk sac. Large, nucleated EryPs begin to circulate around midgestation, when connections between yolk sac and embryonic vasculature mature. Two to 3 days later, small cells of the definitive erythroid lineage (EryD) begin to differentiate within the fetal liver and rapidly outnumber EryPs in the circulation. The development and maturation of EryPs remain poorly defined. Our analysis of embryonic blood at different stages reveals a stepwise developmental progression within the EryP lineage from E9.5 to E12.5. Thereafter, EryDs are also present in the bloodstream, and the 2 lineages are not easily distinguished. We have generated a transgenic mouse line in which the human epsilon-globin gene promoter drives expression of green fluorescent protein exclusively within the EryP lineage. Here, we have used this line to characterize changes in cell morphology and surface-marker expression as EryPs mature and to track EryP numbers and enucleation throughout gestation. This study identifies previously unrecognized synchronous developmental stages leading to the maturation of EryPs in the mouse embryo. Unexpectedly, we find that EryPs are a stable cell population that persists through the end of gestation.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





References
-
- Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. - PubMed
-
- Palis J, Yoder M. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exper Hematol. 2001;29:927–936. - PubMed
-
- Koury MJ, Sawyer ST, Brandt SJ. New insights into erythropoiesis. Curr Opin Hematol. 2002;9:93–100. - PubMed
-
- Miller JL. Hemoglobin switching and modulation: genes, cells, and signals. Curr Opin Hematol. 2002;9:87–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

